Results of intraoperative electron beam radiotherapy containing multimodality treatment for locally unresectable T4 rectal cancer: a pooled analysis of the Mayo Clinic Rochester and Catharina Hospital Eindhoven
Introduction
Treatment of rectal cancer changed significantly over the past decades. The introduction of total mesorectal excision (TME) (1), the evaluation of the circumferential margin (CRM) status (2), the introduction of neoadjuvant (chemo) radiation and adjuvant therapy have improved the prognosis of patients with rectal cancer (3,4).
Rectal carcinomas are considered locally advanced when infiltrating through the enveloping mesorectal fascia or ingrowth into adjacent structures (5). These carcinomas are sub classified as T4b when they adhere to or invade into the surrounding structures or organs according to the TNM classification. The idea of treating patients with these T4 rectal carcinomas that may be locally unresectable for cure at initial presentation has changed from a merely palliative setting to a more aggressive multimodality treatment combining neoadjuvant (chemo) radiation with extended surgery. As a result of this approach the survival also changed from almost no long-term survivors to a reported 5-year survival rate up to 67% (6).
A number of factors appear to influence survival in the treatment of patients with T4b rectal tumors. Among these are the ability to achieve an R0 resection (6-9), and the use of neoadjuvant external beam irradiation therapy (EBRT) or concurrent chemoradiation (CRT) to achieve downsizing and downstaging of the tumour, which may improve the probability of performing a R0 resection. Furthermore neoadjuvant EBRT or CRT improves local control and in some series survival (5,10). However the dose tolerance of normal tissue limits the dose of EBRT that can be delivered safely. The addition of concurrent chemotherapy to the neoadjuvant radiation has improved local control, time to treatment failure, and cancer-specific survival (CSS) vs. EBRT alone in a phase III Norwegian trial of patients with locally unresectable rectal cancer (5).
Intra-operative irradiation therapy with either electrons (IOERT) or high dose brachytherapy (HDR-IORT) can provide a solution to overcome the problem of EBRT dose limitations. With IORT a boost can be delivered to the area of highest risk, i.e., the region where the tumor was initially fixed or most adherent to adjacent structures on clinical exam and confirmed on pelvic imaging and the risk of a close margin or even an R1 or R2 resection is highest. Dose limiting surrounding structures can be removed or shielded from the IORT boost. IORT has become an integral part of multimodality treatment of locally unresectable T4 rectal cancer in a number of institutions worldwide (6,11-30). The combination of EBRT and IORT may result in a tumorcidal dose equivalent to 80–90 Gy in 2 Gy fractions (12).
Two major tertiary referral centres practicing IOERT-containing multimodality treatment in the treatment of locally unresectable primary T4 rectal carcinomas, the Mayo Clinic in Rochester, Minnesota, USA (MAYO) and the Catharina Hospital in Eindhoven, The Netherlands (CHE), pooled their data. The aim of this study was to analyse the patient and treatment factors influencing local recurrence (LR), distant metastases (DM) and survival in uni and multivariate analyses.
Methods
Patients
At the MAYO the IOERT program started in 1981 and since then MAYO has been a leader in the field of treating patients with locally unresectable and recurrent rectal carcinoma (11,13,14,27). The Catharina Hospital Eindhoven (CHE) joined the Mayo Clinic in an IOERT program for rectal cancer in 1994 with the same treatment protocol (15-17).
The clinical stage of all patients was assessed by abdomen-pelvic computed tomography (CT) and or magnetic resonance imaging (MRI) in most patients, demonstrating loss of a fat plane relative to critical organs or structures, and the inability to perform an upfront R0 resection. In addition, patients had routine labs and chest film or CT, and many had endoscopic ultrasound to evaluate depth of invasion.
The data of the patients of the Mayo Clinic and the Catharina Hospital have been pooled from the beginning of their IOERT-program until 2010. Patients with primary T4b rectal cancer, locally unresectable for cure at initial presentation, without pre-operative DM were selected. Patients with a good response to neoadjuvant treatment not necessitating IOERT during surgery were excluded from this analysis resulting in a pooled group of 417 patients. The mean follow up time was 52 months (range, 0–234 months).
Treatment
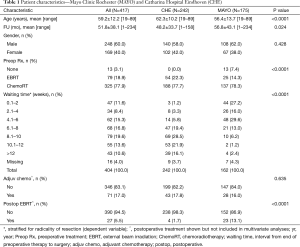
In Table 1 the similarities and differences between the institutions are shown. Patients in the MAYO cohort were significantly younger (mean 56 vs. 62 years in the CHE cohort, P<0.001) and had a longer mean follow up (57 vs. 48 months in the CHE, P=0.009). The preferred treatment approach was preoperative (chemo) radiation, an intended radical resection followed by an IOERT boost at the area of risk, however in the early phase of the MAYO cohort 13 of 175 patients (7.4%) had an irradical surgical resection (R1 or R2) as the initial component of treatment. Over the years, the adjuvant and neoadjuvant treatment schemes have changed in both centers. However, the basic treatment principle (radiotherapy with concurrent 5-fluorouracil or capecitabine, followed by resection and an IOERT boost) remained the same. Treatment methods from both institutions have been described in detail in prior manuscripts and will only be summarized here (13-17,27).
Full table
The majority of the patients received preoperative radiotherapy combined with 5-FU based chemotherapy, and this percentage was comparable in both centers (MCR: 79% vs. CHE: 78%). The preoperative EBRT-dose ranged from 45–54 Gy in fractions of 1.8 to 2.0 Gy.
The waiting period between the finishing of the preoperative (chemo) radiotherapy and surgery ranged from 1 day to more than 12 weeks. At MAYO, the waiting time was significantly shorter than at CHE with a waiting time interval of 6 weeks or less in 73% vs. 10% of patients and an interval of 8 weeks or less in 86% vs. 30%. All patients were re-evaluated, with either a pelvic MRI and/or a CT thorax/abdomen/pelvis, after finishing neoadjuvant therapy and before proceeding to surgery/IOERT.
Performing surgery in these patients constitutes a challenge, given the fact that the tumors were beyond their natural anatomical borders and often required an extra-anatomical resection. IORT was delivered as an electron boost at both institutes. Currently, both centers have a dedicated linear accelerator in the operating theatre. The IOERT dose and energy were comparable and were usually 10 to 12.5 Gy after an R0 or R1 resection (15 Gy or higher in the few patients with R2 resection), with electron energies ranging from 8 to 12 MEV and the most commonly used diameter of the bevelled applicator was 6 cm.
All patients referred for treatment for a T4b carcinoma were discussed in a multidisciplinary setting in both centers. Data of all patients treated for T4 carcinomas are prospectively collected in a database.
Statistical analysis
Statistical analysis was performed using SPSS package (SPSS 20.0 for Windows; SPSS Inc., Chicago, IL). The t-tests and chi-square tests were used to compare individual variables. The LR rate, DM rate, CSS, relapse-free survival (RFS) and overall survival (OS) were estimated using the Kaplan-Meier (KM) method. CSS was defined as the time between rectal cancer surgery and death caused by rectal cancer. Differences were assessed using the Log-Rank test. P values were two-sided and considered statistically significant at a value of 0.05 or less. For determination of risk factors, first univariate analyses were performed by analyzing the effect of the covariates in a univariate Cox regression (CR). Covariates with trend-significant effects (P value <0.10) were selected for multivariate analysis, using stepwise Cox proportional hazards regression modeling. Stepwise regression was used and a two-sided P value of less than 0.05 was considered significant. Forest plots were implemented using Comprehensive Meta-Analysis version 2.0.
In this group of 417 patients only seven R2 resections occurred and therefore, R1 and R2 were combined as R+ in most statistical analyses.
The Mayo Clinic institutional review board and the Catharina Hospital review board approved this study.
Results
Radicality of the resection
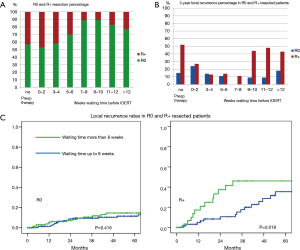
Overall, 306 patients of the 417 patients (73%) underwent a complete resection without tumor cells at the surgical resection margins (R0 resection). Table 2 shows the influence of preoperative parameters on the radicality of the resections. In the group of patients who had no preoperative treatment the R0 rate was 54%, while this ranged from 67% after preoperative radiotherapy and 76% after preoperative chemoradiotherapy (P=0.026). Furthermore it was found that the waiting time between the last day of the neoadjuvant radiotherapy and the day of surgery had a significant effect on the margin status of the resection. In the group of patients who waited less than 57 days (the median number of days waiting time), 69% of patients underwent a R0 resection opposed to 80% in the group of patients who had a longer waiting period (P=0.014). Figure 1A shows the effect of the waiting time on the percentage of R0 resections.

Full table
The LR
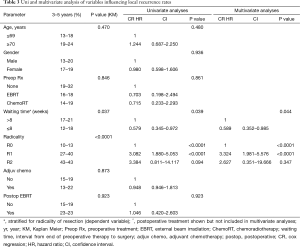
In 66 of 417 patients a LR developed (19.3% 5-year LR rate). After univariate analysis (Table 3) the risk factor associated with LR were margin status of the resection (P<0.001) and interval from completion of preoperative therapy to resection (waiting time; P=0.037). Subtotal resection resulted in 3- and 5-year LR rates of 27% and 40% for R1 resection and 43% for R2 resections, while this was 10% and 13% after R0 resection (P<0.001).
Full table
As waiting time was correlated with radicality of resection, the influence of waiting time on the development of LR was stratified for radicality of resection. After univariate analyses a significant increase in LR was found with a waiting time of >8 vs. ≤8 weeks or less (KM, P=0.037) leading to a risk reduction of 42% (CR, HR: 0.58; CI: 0.35–0.97, P=0.039).
After multivariate analyses with waiting time and radicality of resection included, a significant risk reduction of 41% (CR, HR: 0.59; CI: 0.35–0.99, P=0.044) and 70% (CR, HR: 0.30; CI: 0.18–0.51, P<0.0001) was found for waiting time and radicality of resection respectively. The most prominent risk reduction of almost 60% was seen in the R+ resected group. Figure 1B demonstrates the effect of waiting time on 3 years LR rate in R0 and R+ resected patients. Figure 1C shows the different effect of waiting time on radically resected patients and incomplete resected patients. In R+ patients, the 3-year local relapse rate was 43% vs. 18% with waiting time of >8 vs. ≤8 weeks (P=0.018).
A lower risk of LR was observed at 5 years in patients receiving neoadjuvant therapy, although this effect did not reach statistical significance since 97% of patients received preoperative therapy. No effect of adjuvant chemotherapy was found on the development of a LR.
The DM
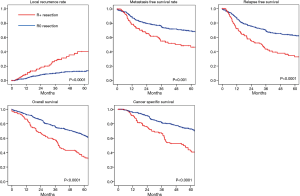
The 5-year DM free survival was 64%. Only an incomplete resection was associated with a higher risk for DM, as shown in Table S1, with 3- and 5-year DM free survival of 76% and 69%, 59% and 47%, and 33% and 33% for R0, R1 and R2 resected patients respectively (P=0.001). When investigating factors influencing the development of metastases, no influence was found of adjuvant therapy in this analysis. Figure 2 shows the effect of an R0 and R+ resection on the development of metastases and other oncological outcome parameters.
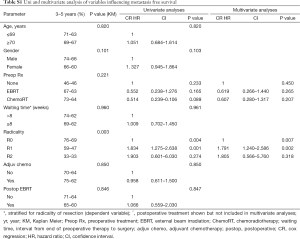
Full table
CSS and relapse-free survival (RFS)
CSS was 64.6% after 5 years; the main factor associated with CSS after univariate analysis was margin status (Table 4). R1-R2 resections were associated with a 3- and 5-year CSS of 69% and 44%, and 40% and 20% respectively, compared to 82% and 73% after R0 surgery (P<0.001). After univariate analysis of factors influencing CSS it was found that receiving preoperative chemoradiotherapy or radiotherapy decreased the chance of dying of the cancer (P=0.036), which did not reach statistical significance on multivariate analysis.
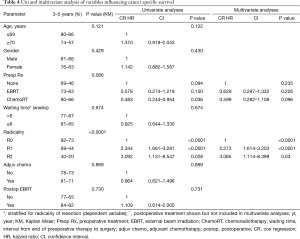
Full table
Five-year RFS was 55.1%. RFS was significantly influenced by the margin status (P<0.001, Table S2). A trend towards improved RFS is observed after neoadjuvant therapy (5-year RFS of 56% vs. 30%, with vs. without neoadjuvant therapy; P=0.148). The RFS was not influenced by adjuvant therapy.
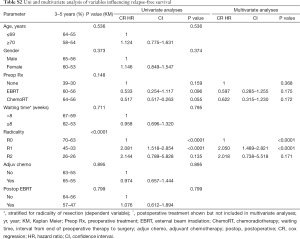
Full table
OS
The 3- and 5-year OS estimates were 73% and 56%. When an R0 resection was achieved, these percentages were 77% 3-year and 64% 5-year OS. In patients with R1-R2 resection the 3 and 5 years OS were 60% and 35%, and 29% and 14% respectively (P<0.001). Factors influencing OS after uni- and multivariate analysis were age (P<0.001) and margin status (P<0.001) (Table 5). The different types of neoadjuvant therapy had no significant effect on survival but the fact that patients did or did not receive neoadjuvant therapy showed a trend towards better survival in patients treated with neoadjuvant therapy (5-year OS of 46% with no preop therapy vs. 57% with preop CRT). Adjuvant chemotherapy resulted in a trend for improved 5-year OS (70% vs. 53%) that did not reach statistical significance (P=0.101). Postoperative radiotherapy was not correlated with OS nor with other oncological outcome parameters
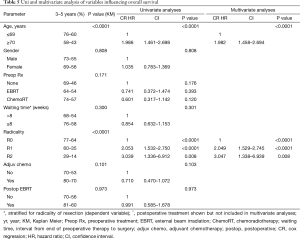
Full table
Discussion
In this study the pooled results of the IOERT containing multimodality treatment in two major centers are presented in 417 patients with T4 rectal carcinomas that were locally unresectable for cure at initial presentation. The pooling of this large cohort of patients with sufficient follow up allowed in-depth statistical analysis. However, combining the data from different centers may also introduce some bias. Therefore the treatment protocols of both centers were compared to evaluate if the groups of patients were comparable and to detect differences, which could be used for analytical purposes. Furthermore the pre-treatment staging and the TNM classification were compared to evaluate if the groups were similar. All patients had a T4b tumor as staged prior to initiation of treatment. The percentage of patients receiving neoadjuvant therapy was comparable in both centers (100% vs. 93%): however, 13 of 175 patients (7.3%) in the early part of the MAYO cohort had an irradical (R1 or R2) resection as the initial component of treatment. Finally specialists of both centers visited each other and observed treatment of patients in the collaborating center. It was concluded that both the patient groups and method of treatment were quite comparable before the pooled analysis was started. Other limitations of the analysis include changes in treatment protocol over time especially with regard to concurrent chemotherapy during EBRT and maintenance postoperative chemotherapy (utilization, which drugs, method of administration, intensity, duration, etc.).
The major difference in the treatment approach at MAYO and CHE was the interval from completion of neoadjuvant therapy to radical resection plus IOERT. At MAYO, the waiting time was 6 weeks or less in 73% of patients vs. 10% at CHE and 8 weeks or less in 86% vs. 30%. The MAYO preferred interval of ≤6 weeks was chosen to obtain an additive effect of the EBRT and IOERT components of treatment by reducing the total duration of irradiation, in an attempt to maximize local control of disease. CHE preferred an interval of 8–10 weeks in order to achieve maximum tumor shrinkage from preoperative therapy and optimize the rate of R0 resection.
The treatment protocols in the current pooled analysis have succeeded in achieving an increased 5-year OS of 56% for patients with T4 rectal carcinomas that were locally unresectable for cure at time of initial presentation because of tumor fixation. The distant metastasis free survival was 64% and CSS was 65% after 5 years. These results compare well with the outcome of rectal cancer treatment in general, when considering that all these patients had ‘locally unresectable’ T4 tumors. Other IOERT series published survival figures ranging from 52% to 67% (6,14,18-20), however some of those studies included T1–T3 tumours (18-20).
In the current pooled analysis the LR rate was 16% (3-year LR: R0—10%, R1—27% and R2—43%). In other IOERT studies similar rates have been reported. Kusters et al. found a LR rate of 12% in a pooled analysis of the results of four European tertiary referral IORT centers treating T4 tumours and T3 tumours with a threatened CRM (6). Mathis et al. analyzed a Mayo IOERT series of 146 patients with unresectable T4 colorectal cancers of which 106 were rectal; the LR rate was 14% (14).
Impact of IOERT on outcomes
It is difficult to assess the effect of adding IOERT to the treatment approach for patients with LARC with regard to increased survival. One would assume that the combination of improved components of the treatment will improve both local control and survival over time, but it is challenging to determine which part of the improvement can be contributed to IOERT. However, in a non-IORT series from MAYO by Schild et al. (24), 17 rectal cancer patients with irradical resection (R1—10 pts, R2—7) received postoperative EBRT or CRT with 3- and 5-year OS of only 24% vs. 5-year OS of 52% in the MAYO IOERT series reported by Mathis et al. (14) and 5-year OS of 56% in the current analysis. In the MAYO non-IORT series of 17 patients, LR occurred in 76% of patients (3 and 5-year LR: resection R1—70%; R2—86%). In the MAYO IOERT series of 146 patients, 3- and 5-year LR rates were 10% and 14%. In the current pooled IOERT analysis, the 5-year LR rate was 19%.
A separate Mayo analysis by Schild et al. was performed regarding outcomes in 103 patients with locally advanced colon cancer treated with EBRT or CRT alone or plus IOERT as a supplement to maximal surgical resection (25). Outcomes at 5-year based on radicality of resection were as follows: LR, resection R0—10%, R1—54%, R2—79% (P<0.0001); 5-year OS, R0—66%, R1—47%, R2—23% (P=0.0009). Patients with R1 or R2 resection who received IOERT plus EBRT/CRT as a component of treatment had better outcomes than those who had only EBRT or CRT: LR—11% vs. 82% (P=0.02); 5-year OS 76% vs. 26% (P=0.04).
In a recent systematic review and meta-analysis evaluating IORT in the treatment of colorectal cancer by Mirnezami et al., it was concluded that IORT may improve oncological outcome in patient treated for LARC (21). The meta-analysis of outcomes for locally advanced primary and recurrent rectal cancer showed a significant effect of IORT for both local control (pooled odds ratio of 0.22, P=0.03) and 5-year OS (HR =0.51, P=0.009) in patients with R0, R1 and R2 resection.
There are no large randomized controlled trials (RCT’s) comparing treatment with or without IOERT in the treatment of T4 rectal cancer that observed a survival benefit for IOERT. Two RCT’s failed to show benefit of the addition IOERT to the treatment of LARC. However in both RCT’s T3 tumors were included, in whom the additional effect of IOERT would theoretically be minimal (18,21,28).
In an analysis from Massachusetts General Hospital (MGH) by Willett et al., outcomes were compared after R0 resection in 20 patients with and 18 patients without IOERT. There was no difference in 5 years disease free survival, but an improved local control rate was found with IOERT (5-year LC of 88% vs. 67%) (22). The improvement in local control with IOERT has been confirmed in a series by Valentini et al. of 78 patients with preoperative chemoradiation (preop CRT) and R0 resection for T4 rectal cancers; 29 had IOERT after resection (29). Local control at 5-year was best in those with IOERT as a component of treatment (100% vs. 81%, P=0.014). On multivariate analysis, IOERT was the only variable with a positive predictive value.
In a separate MGH series by Willett et al., 47 patients with locally advanced rectal cancer had preop CRT and R0 resection, but IOERT was not given (good tumor response or not technically feasible) (30). In patients with a pathological CR or ypT2N0 disease, 5-year LR was only 13%, but in those with ypT3N0 or nodal disease, 5-year LR was 68%. In a subsequent analysis by Willett et al., 95 patients with T4 rectal cancer had preop EBRT/CRT followed by complete resection; 40 had IOERT and 55 did not (favorable tumor response or IOERT not feasible technically) (31). Local control was better in IOERT patients, in both responders (100% vs. 84%) and non-responders (88% vs. 73%). In view of these findings, the authors recommended that IORT should be delivered, if technically feasible, independent of the extent of tumor downstaging after preoperative treatment.
In the aforementioned study by Kusters it was found that 55% of patients treated with IOERT for positive resection margins had no LR. A similar observation was noted by Mathis et al., who found that only 2% of subsequent LRs were located in the IOERT field (14). When an R0 resection is not feasible, Ferenschild et al. found an improved local control rate and OS with the addition of HDR-IORT with 5-year local control of 58% vs. 0% (23).
In a recent Memorial Sloan Kettering HDR-IORT analysis by Terezakis et al., 89 patients with T4 rectal cancer had preoperative chemoradiation followed by R0 or R1 resection and HDR-IORT (HDR-IORT not feasible after R2 resection) (26). Outcomes were similar to IOERT series with 5-year LR of 23%, 26% and 17% for negative (n=58), close (2 mm or less; n=16) or positive R1 resection margins (n=16) and 5-year OS of 57%, 60% and 45% respectively.
All these observations may lead to the assumption that IORT with either electrons or HDR brachytherapy has an effect on residual tumor cells. This effect has the potential to impact both local control and survival.
Waiting time—impact on outcomes
A new finding in this pooled analysis was that a relatively short waiting time between the last day of preoperative radio (chemo) therapy and the administration of IOERT was important to reduce LR. Patients with a waiting time of >8 weeks had a higher rate of local relapse than those with a waiting time of 8 weeks or less (P=0.044, multivariate). The impact of waiting time on LR extended from 3 to 8 weeks following completion of neoadjuvant treatment. From a radio-biological point of view this finding seems logical: the longer the waiting time the more effective the repopulation of cancer cells in the previously irradiated tumor.
In a prospective randomized study evaluating the optimal waiting period in patients with rectal carcinomas (T2 to T3, NX, M0) after neoadjuvant radiation therapy it was concluded that a waiting period of 6 weeks is optimal, mainly because tumour downsizing was increased and no detrimental effects on toxicity and early clinical results was observed. No difference in LR or short-term survival was found in this study (32). However, in patients with locally unresectable rectal cancer, who are treated with neoadjuvant concurrent chemoradiation, no results of prospective studies evaluating the optimal waiting period are available. In patients with rectal carcinomas retrospective data is available on the subject of a longer waiting period after finishing the neoadjuvant chemoradiation. Results have been published that a longer period waiting after neoadjuvant chemoradiation accomplishes a higher level of pathological complete response (pCR) (33) or more downstaging (34,35), without an increase in complications. However an increase in disease free or OS has not been shown.
In LARC patients, an analysis by Tulchinsky et al. showed that an interval between neoadjuvant chemoradiation and surgery of more than 7 weeks was associated with higher rates of pCR and near pCR, decreased recurrence and disease free survival in 132 patients analysed (36). de Campos-Lobato et al. evaluated the same subject in LARC patients with an interval shorter or longer than 8 weeks. They also found a significant higher rate of complete response (P=0.03) and a (not significant) correlation with decreased LR (P=0.07) (37). Other studies confirmed the effect of longer waiting on downstaging, but did not find an effect on survival (34,38).
However, other studies did not find an effect of a longer waiting period. Stein et al. evaluated downstaging in LARC patients divided in two groups (a waiting time of 4–8 and 10–14 weeks) They found no influence of longer waiting on perioperative morbidity and downstaging (39). A similar outcome was found in a Korean study by Lim et al. which was performed to evaluate the optimal waiting time to LARC surgery after preoperative chemoradiation (CRT) to 50.4 Gy with resection 4–8 weeks later (40). There was no difference in pathologic or surgical outcomes in those who had surgery 28–41 d after CRT vs. 42–56 d (pCR—13.8% vs. 15.0%; downstaging—47.5% vs. 44.4%; sphincter preservation—83.9% vs. 82%) and both groups had similar local-recurrence free survival (P=0.1165).
In the current pooled analysis, longer waiting times did have a significant effect on the R0 rate, which was an important factor influencing LR, DM, CSS, RFS and OS. The highest radicality rate was achieved with the waiting period of 6–10 weeks (Figure 1). However, waiting times longer than eight weeks resulted in an increased rate of LR in both univariate analyses (P=0.037) and multivariate analyses (P=0.044) which may indicate the lack of an additive effect of IOERT to preoperative EBRT or chemoradiation with the longer waiting times. In fact, waiting times longer than twelve weeks resulted in LR rates comparable to no preoperative radio (-chemo) therapy. Accordingly, in institutions without the capability of an IORT boost after radical resection, the waiting time can and perhaps should be tailored to the tumor characteristics in the individual patient. If a longer waiting is expected to create downsizing of the tumour leading to a situation in which a radical resection can be performed more easily, it may be preferable to wait longer than six weeks to get the maximum effect of the neoadjuvant therapy. However, in institutions with IORT capability, it may be preferable to perform the resection after a 3–8-week interval, to increase the likelihood of an additive effect of IORT plus EBRT in destroying residual tumour cells.
Adjuvant chemotherapy
In the current pooled analysis, 79 patients (19%) received post-op chemotherapy with no impact on disease relapse or survival. Previous reports on the role of adjuvant chemotherapy concluded that the addition of adjuvant chemotherapy in the treatment of patients with colorectal carcinoma is beneficial in selected patients (41,42). A more recent analysis of the phase III EORTC 2291 trial in 1,011 patients with stage II or III rectal cancer confirms the benefit of preoperative concurrent chemoradiation vs. preop EBRT alone with regard to improved local control, but no survival benefit was found for adjuvant postop chemotherapy (43,44).
Conclusions
In conclusion, an acceptable disease free and OS can be achieved in treatment of patients with locally unresectable T4b rectal cancer with a combined modality regimen that includes neoadjuvant chemoradiation, radical intent surgery and IORT. Radicality of the resection has a significant impact on all oncological outcome parameters. Early administration of an IORT boost in margin positive and probably also margin close patients may reduce the development of LRs by more than 50% and result in LR rates comparable to radically resected patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Both the Catharina Hospital and the Mayo Clinic did not require formal approval of the ethical committees nor informed consent because data collected and provided were completely anonymous and the study without intervention did not violate the privacy of individual patients.
References
- Heald RJ. The ‘Holy Plane’ of rectal surgery. J R Soc Med 1988;81:503-8. [PubMed]
- Glynne-Jones R, Mawdsley S, Novell JR. The clinical significance of the circumferential resection margin following preoperative pelvic chemo-radiotherapy in rectal cancer: why we need a common language. Colorectal Dis 2006;8:800-7. [Crossref] [PubMed]
- Bosset JF, Calais G, Mineur L, et al. Enhanced tumorocidal effect of chemotherapy with preoperative radiotherapy for rectal cancer: preliminary results--EORTC 22921. J Clin Oncol 2005;23:5620-7. [Crossref] [PubMed]
- Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731-40. [Crossref] [PubMed]
- Braendengen M, Tveit KM, Berglund A, et al. Randomized phase III study comparing preoperative radiotherapy with chemoradiotherapy in nonresectable rectal cancer. J Clin Oncol 2008;26:3687-94. [Crossref] [PubMed]
- Kusters M, Valentini V, Calvo FA, et al. Results of European pooled analysis of IORT-containing multimodality treatment for locally advanced rectal cancer: adjuvant chemotherapy prevents local recurrence rather than distant metastases. Ann Oncol 2010;21:1279-84. [Crossref] [PubMed]
- Nagtegaal ID, Quirke P. What is the role for the circumferential margin in the modern treatment of rectal cancer? J Clin Oncol 2008;26:303-12. [Crossref] [PubMed]
- Trakarnsanga A, Gonen M, Shia J, et al. What is the significance of the circumferential margin in locally advanced rectal cancer after neoadjuvant chemoradiotherapy? Ann Surg Oncol 2013;20:1179-84. [Crossref] [PubMed]
- Tilney HS, Rasheed S, Northover JM, et al. The influence of circumferential resection margins on long-term outcomes following rectal cancer surgery. Dis Colon Rectum 2009;52:1723-9. [Crossref] [PubMed]
- Aklilu M, Eng C. The current landscape of locally advanced rectal cancer. Nat Rev Clin Oncol 2011;8:649-59. [Crossref] [PubMed]
- Gunderson LL, Haddock MG, Nelson H, et al. Locally recurrent colorectal cancer: IOERT and EBRT +/-5-FU and maximal resection. Front Radiat Ther Oncol 1997;31:224-8. [Crossref] [PubMed]
- Willett CG, Czito BG, Tyler DS. Intraoperative radiation therapy. J Clin Oncol 2007;25:971-7. [Crossref] [PubMed]
- Gunderson LL, Martin JK, Beart RW, et al. Intraoperative and external beam irradiation for locally advanced colorectal cancer. Ann Surg 1988;207:52-60. [Crossref] [PubMed]
- Mathis KL, Nelson H, Pemberton JH, et al. Unresectable colorectal cancer can be cured with multimodality therapy. Ann Surg 2008;248:592-8. [PubMed]
- Mannaerts GH, Martijn H, Crommelin MA, et al. Feasibility and first results of multimodality treatment, combining EBRT, extensive surgery, and IOERT in locally advanced primary rectal cancer. Int J Radiat Oncol Biol Phys 2000;47:425-33. [Crossref] [PubMed]
- Rutten HJ, Mannaerts GH, Martijn H, et al. Intraoperative radiotherapy for locally recurrent rectal cancer in The Netherlands. Eur J Surg Oncol 2000;26 Suppl A:S16-20.
- Mannaerts GH, Schijven MP, Hendrikx A, et al. Urologic and sexual morbidity following multimodality treatment for locally advanced primary and locally recurrent rectal cancer. Eur J Surg Oncol 2001;27:265-72. [Crossref] [PubMed]
- Dubois JB, Bussieres E, Richaud P, et al. Intra-operative radiotherapy of rectal cancer: results of the French multi-institutional randomized study. Radiother Oncol 2011;98:298-303. [Crossref] [PubMed]
- Sadahiro S, Suzuki T, Ishikawa K, et al. Preoperative radio/chemo-radiotherapy in combination with intraoperative radiotherapy for T3-4Nx rectal cancer. Eur J Surg Oncol 2004;30:750-8. [Crossref] [PubMed]
- Diaz-Gonzalez JA, Calvo FA, Cortes J, et al. Prognostic factors for disease-free survival in patients with T3-4 or N+ rectal cancer treated with preoperative chemoradiation therapy, surgery, and intraoperative irradiation. Int J Radiat Oncol Biol Phys 2006;64:1122-8. [Crossref] [PubMed]
- Mirnezami R, Chang GJ, Das P, et al. Intraoperative radiotherapy in colorectal cancer: systematic review and meta-analysis of techniques, long-term outcomes, and complications. Surg Oncol 2013;22:22-35. [Crossref] [PubMed]
- Willett CG, Shellito PC, Tepper JE, et al. Intraoperative electron beam radiation therapy for primary locally advanced rectal and rectosigmoid carcinoma. J Clin Oncol 1991;9:843-9. [PubMed]
- Ferenschild FT, Vermaas M, Nuyttens JJ, et al. Value of intraoperative radiotherapy in locally advanced rectal cancer. Dis Colon Rectum 2006;49:1257-65. [Crossref] [PubMed]
- Schild SE, Martenson JA Jr, Gunderson LL, et al. Long-term survival and patterns of failure after postoperative radiation therapy for subtotally resected rectal adenocarcinoma. Int J Radiat Oncol Biol Phys 1989;16:459-63. [Crossref] [PubMed]
- Schild SE, Gunderson LL, Haddock MG, et al. The treatment of locally advanced colon cancer. Int J Radiat Oncol Biol Phys 1997;37:51-8. [Crossref] [PubMed]
- Terezakis S, Morikawa L, Wu A, et al. Long-Term Survival After High-Dose-Rate Brachytherapy for Locally Advanced or Recurrent Colorectal Adenocarcinoma. Ann Surg Oncol 2015;22:2168-78. [Crossref] [PubMed]
- Gunderson LL, Nelson H, Martenson JA, et al. Locally advanced primary colorectal cancer: intraoperative electron and external beam irradiation +/- 5-FU. Int J Radiat Oncol Biol Phys 1997;37:601-14. [Crossref] [PubMed]
- Masaki T, Takayama M, Matsuoka H, et al. Intraoperative radiotherapy for oncological and function-preserving surgery in patients with advanced lower rectal cancer. Langenbecks Arch Surg 2008;393:173-80. [Crossref] [PubMed]
- Valentini V, Coco C, Rizzo G, et al. Outcomes of clinical T4M0 extra-peritoneal rectal cancer treated with preoperative radiochemotherapy and surgery: a prospective evaluation of a single institutional experience. Surgery 2009;145:486-94. [Crossref] [PubMed]
- Willett CG, Shellito PC, Gunderson LL. Primary colorectal EBRT and IOERT. Humana Press 1999;249-72.
- Willett CG, Nakfoor BK, Daley W, et al. Pathological downstaging does not guide the need for IORT in primary locally advanced rectal cancer. Front Radiat Ther Oncol 1997;31:245-6. [Crossref] [PubMed]
- Francois Y, Nemoz CJ, Baulieux J, et al. Influence of the interval between preoperative radiation therapy and surgery on downstaging and on the rate of sphincter-sparing surgery for rectal cancer: the Lyon R90-01 randomized trial. J Clin Oncol 1999;17:2396. [PubMed]
- Kalady MF, de Campos-Lobato LF, Stocchi L, et al. Predictive factors of pathologic complete response after neoadjuvant chemoradiation for rectal cancer. Ann Surg 2009;250:582-9. [PubMed]
- Evans J, Tait D, Swift I, et al. Timing of surgery following preoperative therapy in rectal cancer: the need for a prospective randomized trial? Dis Colon Rectum 2011;54:1251-9. [Crossref] [PubMed]
- Garcia-Aguilar J, Smith DD, Avila K, et al. Optimal timing of surgery after chemoradiation for advanced rectal cancer: preliminary results of a multicenter, nonrandomized phase II prospective trial. Ann Surg 2011;254:97-102. [Crossref] [PubMed]
- Tulchinsky H, Shmueli E, Figer A, et al. An interval >7 weeks between neoadjuvant therapy and surgery improves pathologic complete response and disease-free survival in patients with locally advanced rectal cancer. Ann Surg Oncol 2008;15:2661-7. [Crossref] [PubMed]
- de Campos-Lobato LF, Geisler DP, da Luz Moreira A, et al. Neoadjuvant therapy for rectal cancer: the impact of longer interval between chemoradiation and surgery. J Gastrointest Surg 2011;15:444-50. [Crossref] [PubMed]
- Dolinsky CM, Mahmoud NN, Mick R, et al. Effect of time interval between surgery and preoperative chemoradiotherapy with 5-fluorouracil or 5-fluorouracil and oxaliplatin on outcomes in rectal cancer. J Surg Oncol 2007;96:207-12. [Crossref] [PubMed]
- Stein DE, Mahmoud NN, Anne PR, et al. Longer time interval between completion of neoadjuvant chemoradiation and surgical resection does not improve downstaging of rectal carcinoma. Dis Colon Rectum 2003;46:448-53. [Crossref] [PubMed]
- Lim SB, Choi HS, Jeong SY, et al. Optimal surgery time after preoperative chemoradiotherapy for locally advanced rectal cancers. Ann Surg 2008;248:243-51. [Crossref] [PubMed]
- Collette L, Bosset JF, den Dulk M, et al. Patients with curative resection of cT3-4 rectal cancer after preoperative radiotherapy or radiochemotherapy: does anybody benefit from adjuvant fluorouracil-based chemotherapy? A trial of the European Organisation for Research and Treatment of Cancer Radiation Oncology Group. J Clin Oncol 2007;25:4379-86. [Crossref] [PubMed]
- Gray R, Barnwell J, McConkey C, et al. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet 2007;370:2020-9. [Crossref] [PubMed]
- Bosset JF, Calais G, Mineur L, et al. Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: long-term results of the EORTC 22921 randomised study. Lancet Oncol 2014;15:184-90. [Crossref] [PubMed]
- Hong TS, Ryan DP. Adjuvant Chemotherapy for Locally Advanced Rectal Cancer: Is It a Given? J Clin Oncol 2015;33:1878-80. [Crossref] [PubMed]