Pretreatment tumor volume as a prognostic factor in metastatic colorectal cancer treated with selective internal radiation to the liver using yttrium-90 resin microspheres
Introduction
The liver is the most common site for metastases in patients with colorectal cancer (CRC), either at the time of diagnosis or later in the course of disease (1,2). Although long-term survival and even cure are achievable with complete resection of both the primary tumor and distant metastases, only 10–20% of patients are suitable surgical candidates––for multiple reasons, including large tumor burden, multifocal disease, and inadequate liver function (2-5). Recent improvements in chemotherapy and targeted biologic agents have improved survival for patients with metastatic CRC, but overall survival (OS) remains closely tied to liver tumor burden. A complete response within the liver from systemic therapy alone is uncommon; therefore, liver-directed therapies may be considered in select patients with liver metastases to enhance intrahepatic disease control, preserve normal liver function, and potentially prolong survival.
The normal liver is one of the most radiosensitive organs. University of Michigan investigators demonstrated that although the whole liver can tolerate only low doses, increasing doses can be safely delivered to decreasing liver volume (6). Techniques such as stereotactic body radiation therapy and selective internal radiation therapy (SIRT) can deliver a high tumoricidal dose while significantly sparing normal liver. SIRT delivers beta-emitting yttrium-90 (90Y) microspheres to liver tumors through the hepatic arterial system and is able to largely spare the normal liver because of the short distance over which radiation is emitted from each microsphere.
SIRT has been studied as first-line therapy for hepatic colorectal metastases (7-10), in combination with second- or third-line chemotherapy (11,12), and as salvage therapy for chemorefractory patients (13-18). These studies have been important in demonstrating that SIRT can prolong intrahepatic disease control and improve OS.
Several of these studies have investigated prognostic factors for improved outcomes in SIRT, including age, extrahepatic disease, performance status, and carcinoembryonic antigen levels (10,14,19,20). However, the relationship between pre-SIRT liver tumor volume independent of total number of liver lesions and outcomes has not well described.
Methods
This IRB-approved retrospective review included all patients with metastatic CRC who were treated with 90Y-resin microspheres between 2004 and 2012 at our institution. Each patient was required to have undergone MR or CT imaging of the liver with intravenous (IV) contrast as well as liver function tests prior to SIRT. SIRT candidates underwent hepatic angiography and nuclear imaging utilizing 99mTc-labeled albumin to identify the degree of hepatopulmonary shunting. Patients deemed suitable for SIRT then underwent treatment. Patients with bilobar disease were treated in either sequential lobar or whole-liver fashion. All patients included in the study had to have undergone follow-up CT or MR imaging of the liver with IV contrast ~2 months after the last SIRT, using the same imaging modality as before SIRT. A majority of patients also underwent a follow-up CT or MR scan of the liver with IV contrast at ~5 and 8 months after SIRT.
All post-treatment images were transferred into our treatment planning system (Pinnacle, Philips, Andover, MA). Each metastatic liver lesion was contoured on all post-treatment scans. The volume of each lesion was summed to determine the total liver tumor volume (cc) at a given time point. Previously published volumetric response criteria were utilized and described as follows: complete response (tumor disappearance), partial response (>65% reduction in volume), stable disease (≤65% reduction, ≤44% increase), and progressive disease (>44% increase) (21,22).
Kaplan-Meier analyses were performed on the following categorical variables: sex, number of lesions (<5 or ≥5 lesions), KRAS mutation status, pretreatment volume by quartiles, pretreatment volume by thirds, volumetric response at first follow-up, volumetric response at second follow-up, and volumetric response at third follow-up. The relationship between pre-SIRT tumor volume and radiographic treatment response by three-dimensional volumetric criteria after ~2, 5, and 8 months was evaluated by logistic regression analysis. Prior to modeling, the pretreatment volume was log transformed to reduce variance.
A cutoff point analysis for the pretreatment volume was also performed. The optimal cut point was calculated with the online tool, Cutoff Finder (Version 2.15.0, Charité–Universitätsmedizin Berlin, Berlin, Germany), as described in Budczies et al. (23). The log-rank test statistic was used to estimate the cut point that maximized the difference in volumetric treatment response between subjects in the two groups defined by the cut point, according to the method of Contal and O’Quigley (24).
Results
Sixty patients with a median age of 59 years (range, 38–97 years) were included in this review. Patient demographics and characteristics are detailed in Table 1. Patients received a median of two cycles of chemotherapy prior to SIRT, and median pretreatment volume was 77 cc (range, 4.5–2,170.4 cc). Thirty percent of patients had <5 metastatic lesions, and 25% had the KRAS wild-type mutation. Approximately 72% patients had no extrahepatic metastases, and 70% patients received sequential lobar treatment. Median follow-up from first SIRT was 8.9 months. Pre- and post-SIRT tumor volumes were primarily calculated on CT data (87%). No patient had a radiographic complete response.
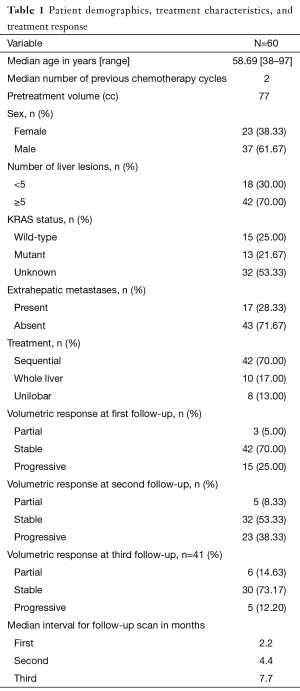
Full table
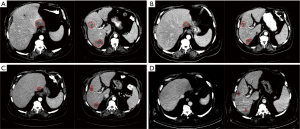
Figure 1 is an example of a patient with partial response to SIRT on first, second, and third follow-up scans. The patient received one treatment to the whole liver. The patient had >5 metastatic liver lesions, and the pre-SIRT volume was 140 cc. Tumor volume decreased to 40, 23.8, and 8.6 cc at first, second, and third follow-ups, respectively.
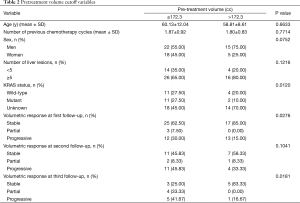
For the cutoff analysis for pretreatment volume, the optimal cutoff point was 172.33 cc. Table 2 shows that volumetric responses at first and third follow-ups were statistically significant, as was KRAS mutation status.
Full table
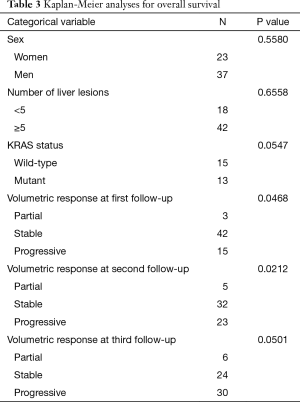
The Kaplan-Meier analysis showed that volumetric responses at first and second follow-ups were statistically significant, whereas volumetric response at third follow-up and KRAS mutation status showed a trend for OS (Table 3). On multivariate analysis, only KRAS status continued to show a trend for OS (P=0.07). Although the sample size for the partial response group was small, so that the results must be interpreted with caution, one can extrapolate that volumetric response may be prognostic of OS.
Full table
Pretreatment volume was a significant predictor for estimating the odds of a patient having stable disease or partial response using volumetric response criteria at first (P=0.0158), second (P=0.0226), and third (P=0.0146) follow-up. For each unit increase in log volume, a patient’s odds of having a stable or partial response were 0.565, 0.630, and 0.606 times as likely at first, second, and third follow-up, respectively.
Discussion
Although pretreatment volume was not significantly associated with OS, it was a significant predictor for treatment response at all three follow-up intervals (~2.2, 4.4, and 7.7 months) following SIRT. Our data also show that patients with metastatic CRC with a larger overall pretreatment liver tumor volume, regardless of number of individual liver lesions, were less likely to have stable or partial responses to SIRT.
Our study is unique in that we evaluated 3-dimensional volumetric assessment. The limitations of unidimensional Response Evaluation Criteria in Solid Tumors (RECIST) and bidimensional World Health Organization (WHO) criteria are well recognized (25,26). RECIST and WHO criteria estimate overall tumor volume assuming tumors to be spherical in shape (27); however, this is clearly not the case for malignant lesions and can make observer reproducibility difficult (28). Erasmus et al. compared unidimensional and bidimensional measurements for 33 lung cancer patients by five different radiologists and found significant differences among readers, leading to incorrect interpretation of tumor response (29).
Today, when patients typically undergo pretreatment CT imaging for SIRT, it is understandable for clinicians to take advantage of 3D imaging data to better determine tumor response as well as provide improved reproducibility. Gordon et al. found reliable reproducibility with MR-based volume measurements of squamous cell carcinoma of the pharynx (30). Zhao et al. found that volumetric measurement allowed for a larger number of lung cancer patients to be identified with absolute changes in tumor volume of at least 20% and 30% compared to uni- and bi-dimensional measurement, respectively (31). Warren et al. compared all three methods in evaluating tumor response in childhood brain tumors (32). Although no differences in detection of partial response were noted, median time to progression was shorter using the 3D method, implying that the method used to assess tumor progression may influence determination of progression-free survival in this cohort of patients (33).
The prognostic value of pretreatment volume is not well described in the current literature. Bester et al. performed a retrospective review of 319 patients with metastatic CRC who underwent 90Y SIRT for chemorefractory liver metastases (17). On multivariate analysis, the authors found that the extent of hepatic disease (≤25% vs. >25%), in addition to use of SIRT and previous chemotherapy, was prognostic. CT hepatic angiography was used to determine extent of hepatic disease (17). The extent of hepatic disease was also found to be prognostic on univariate analysis by Stubbs et al. (33); in contrast, Jakobs et al. did not find the extent of hepatic disease to be prognostic on univariate analysis (34). Weng et al. evaluated largest tumor size among other clinical factors but did not find it to be prognostic (35). We were unable to find other studies that evaluated pretreatment volume as a prognostic factor for survival or predictive factor for response.
One possible explanation for our data showing pretreatment volume to be predictive of response but not of survival is that CT imaging may not be indicative of viable tumor; factors such as necrosis may confound the true tumor volume. Post-treatment CT changes, such as edema, hemorrhage, and ring enhancement, can also confound assessment of tumor response. Although we were able to demonstrate that pretreatment volume was predictive of response using volumetric criteria, our results might have been improved by the use of metabolic imaging, including 18F-FDG PET or functional MR imaging with diffusion-weighted sequences (14,20).
In conclusion, pretreatment volume was a significant predictor for treatment response at all three follow-ups (~2.2, 4.4, and 7.7 months) after SIRT. Our data also show that patients with metastatic CRC with larger overall pretreatment liver tumor volumes, regardless of number of individual liver lesions, were less likely to have radiographic evidence of stable disease or partial response following SIRT. However, pretreatment volume was not significantly associated with OS; thus SIRT is still a reasonable treatment option for patients with extensive pretreatment volumetric tumor burden.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Disclaimer: This paper was presented at the 2014 American Society for Radiation Oncology annual meeting.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Chong G, Cunningham D. Improving long-term outcomes for patients with liver metastases from colorectal cancer. J Clin Oncol 2005;23:9063-6. [Crossref] [PubMed]
- Cummings LC, Payes JD, Cooper GS. Survival after hepatic resection in metastatic colorectal cancer: a population-based study. Cancer 2007;109:718-26. [Crossref] [PubMed]
- Rees M, Tekkis PP, Welsh FK, et al. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg 2008;247:125-35. [Crossref] [PubMed]
- Morris EJ, Forman D, Thomas JD, et al. Surgical management and outcomes of colorectal cancer liver metastases. Br J Surg 2010;97:1110-8. [Crossref] [PubMed]
- Dawson LA, Normolle D, Balter JM, et al. Analysis of radiation-induced liver disease using the Lyman NTCP model. Int J Radiat Oncol Biol Phys 2002;53:810-21. [Crossref] [PubMed]
- Gray B, Van Hazel G, Hope M, et al. Randomised trial of SIR-Spheres plus chemotherapy vs. chemotherapy alone for treating patients with liver metastases from primary large bowel cancer. Ann Oncol 2001;12:1711-20. [Crossref] [PubMed]
- van Hazel GA, Pavlakis N, Goldstein D, et al. Treatment of fluorouracil-refractory patients with liver metastases from colorectal cancer by using yttrium-90 resin microspheres plus concomitant systemic irinotecan chemotherapy. J Clin Oncol 2009;27:4089-95. [Crossref] [PubMed]
- Sharma RA, Van Hazel GA, Morgan B, et al. Radioembolization of liver metastases from colorectal cancer using yttrium-90 microspheres with concomitant systemic oxaliplatin, fluorouracil, and leucovorin chemotherapy. J Clin Oncol 2007;25:1099-106. [Crossref] [PubMed]
- Kosmider S, Tan TH, Yip D, et al. Radioembolization in combination with systemic chemotherapy as first-line therapy for liver metastases from colorectal cancer. J Vasc Interv Radiol 2011;22:780-6. [Crossref] [PubMed]
- Van Hazel G, Blackwell A, Anderson J, et al. Randomised phase 2 trial of SIR-Spheres plus fluorouracil/leucovorin chemotherapy versus fluorouracil/leucovorin chemotherapy alone in advanced colorectal cancer. J Surg Oncol 2004;88:78-85. [Crossref] [PubMed]
- Lim L, Gibbs P, Yip D, et al. A prospective evaluation of treatment with Selective Internal Radiation Therapy (SIR-spheres) in patients with unresectable liver metastases from colorectal cancer previously treated with 5-FU based chemotherapy. BMC Cancer 2005;5:132. [Crossref] [PubMed]
- Kennedy AS, Coldwell D, Nutting C, et al. Resin 90Y-microsphere brachytherapy for unresectable colorectal liver metastases: modern USA experience. Int J Radiat Oncol Biol Phys 2006;65:412-25. [Crossref] [PubMed]
- Cianni R, Urigo C, Notarianni E, et al. Selective internal radiation therapy with SIR-spheres for the treatment of unresectable colorectal hepatic metastases. Cardiovasc Intervent Radiol 2009;32:1179-86. [Crossref] [PubMed]
- Cosimelli M, Golfieri R, Cagol PP, et al. Multi-centre phase II clinical trial of yttrium-90 resin microspheres alone in unresectable, chemotherapy refractory colorectal liver metastases. Br J Cancer 2010;103:324-31. [Crossref] [PubMed]
- Hendlisz A, Van den Eynde M, Peeters M, et al. Phase III trial comparing protracted intravenous fluorouracil infusion alone or with yttrium-90 resin microspheres radioembolization for liver-limited metastatic colorectal cancer refractory to standard chemotherapy. J Clin Oncol 2010;28:3687-94. [Crossref] [PubMed]
- Bester L, Meteling B, Pocock N, et al. Radioembolization versus standard care of hepatic metastases: comparative retrospective cohort study of survival outcomes and adverse events in salvage patients. J Vasc Interv Radiol 2012;23:96-105. [Crossref] [PubMed]
- Evans KA, Richardson MG, Pavlakis N, et al. Survival outcomes of a salvage patient population after radioembolization of hepatic metastases with yttrium-90 microspheres. J Vasc Interv Radiol 2010;21:1521-6. [Crossref] [PubMed]
- Sato KT, Lewandowski RJ, Mulcahy MF, et al. Unresectable chemorefractory liver metastases: radioembolization with 90Y microspheres--safety, efficacy, and survival. Radiology 2008;247:507-15. [Crossref] [PubMed]
- Martin LK, Cucci A, Wei L, et al. Yttrium-90 radioembolization as salvage therapy for colorectal cancer with liver metastases. Clin Colorectal Cancer 2012;11:195-9. [Crossref] [PubMed]
- Chuong MD, Hayman TJ, Patel MR, et al. Comparison of 1-, 2-, and 3-Dimensional Tumor Response Assessment After Neoadjuvant GTX-RT in Borderline-Resectable Pancreatic Cancer. Gastrointest Cancer Res 2011;4:128-34. [PubMed]
- Luccichenti G, Cademartiri F, Sianesi M, et al. Radiologic assessment of rectosigmoid cancer before and after neoadjuvant radiation therapy: comparison between quantitation techniques. AJR Am J Roentgenol 2005;184:526-30. [Crossref] [PubMed]
- Budczies J, Klauschen F, Sinn BV, et al. Cutoff Finder: a comprehensive and straightforward Web application enabling rapid biomarker cutoff optimization. PLoS One 2012;7:e51862. [Crossref] [PubMed]
- Contal C, O'Quigley J. An application of changepoint methods in studying the effect of age on survival in breast cancer. Comput Stat Data An 1999;30:253-70. [Crossref]
- Yaghmai V, Miller FH, Rezai P, et al. Response to treatment series: part 2, tumor response assessment--using new and conventional criteria. AJR Am J Roentgenol 2011;197:18-27. [Crossref] [PubMed]
- Choi H, Charnsangavej C, de Castro Faria S, et al. CT evaluation of the response of gastrointestinal stromal tumors after imatinib mesylate treatment: a quantitative analysis correlated with FDG PET findings. AJR Am J Roentgenol 2004;183:1619-28. [Crossref] [PubMed]
- Cademartiri F, Luccichenti G, Maffei E, et al. Imaging for oncologic staging and follow-up: review of current methods and novel approaches. Acta Biomed 2008;79:85-91. [PubMed]
- Jaffe CC. Measures of response: RECIST, WHO, and new alternatives. J Clin Oncol 2006;24:3245-51. [Crossref] [PubMed]
- Erasmus JJ, Gladish GW, Broemeling L, et al. Interobserver and intraobserver variability in measurement of non-small-cell carcinoma lung lesions: implications for assessment of tumor response. J Clin Oncol 2003;21:2574-82. [Crossref] [PubMed]
- Gordon AR, Loevner LA, Shukla-Dave A, et al. Intraobserver variability in the MR determination of tumor volume in squamous cell carcinoma of the pharynx. AJNR Am J Neuroradiol 2004;25:1092-8. [PubMed]
- Zhao B, Schwartz LH, Moskowitz CS, et al. Lung cancer: computerized quantification of tumor response--initial results. Radiology 2006;241:892-8. [Crossref] [PubMed]
- Warren KE, Patronas N, Aikin AA, et al. Comparison of one-, two-, and three-dimensional measurements of childhood brain tumors. J Natl Cancer Inst 2001;93:1401-5. [Crossref] [PubMed]
- Stubbs RS, O'Brien I, Correia MM. Selective internal radiation therapy with 90Y microspheres for colorectal liver metastases: single-centre experience with 100 patients. ANZ J Surg 2006;76:696-703. [Crossref] [PubMed]
- Jakobs TF, Hoffmann RT, Dehm K, et al. Hepatic yttrium-90 radioembolization of chemotherapy-refractory colorectal cancer liver metastases. J Vasc Interv Radiol 2008;19:1187-95. [Crossref] [PubMed]
- Weng Z, Ertle J, Zheng S, et al. A new model to estimate prognosis in patients with hepatocellular carcinoma after Yttrium-90 radioembolization. PLoS One 2013;8:e82225. [Crossref] [PubMed]


