Treatment of non-resectable and metastatic gastrointestinal stromal tumors: experience with the use of tyrosine kinase inhibitors in a third level hospital in Mexico
Introduction
Stromal tumors that affect the gastrointestinal tract are uncommon, representing about 1% of the overall cancer cases. Clinically, they present as subepithelial lesions. The group of highest frequency is formed by the gastrointestinal stromal tumors (GIST). The common locations for this tumor are gastric and small bowel level, however, they may develop at any portion of the digestive tract including the omentum, mesenterium, and peritoneum (1).
At the molecular level, this type of tumors are characterized by the expression of CD117, part of the KIT receptor, a membrane kinase produced by the KIT proto-oncogene, this expression has been reported in up to 80% of the GIST cases, however, there are mutations related to the over expression of other tyrosine kinase receptors, such as the platelet derived growth factor receptor alpha (PDGFRA) (2).
The treatment assays based on chemotherapy have demonstrated low efficacy in the management of non-resectable and/or metastatic tumors, showing a high rate of primary resistance to this kind of management (3).
The treatment of this type of patients experienced a radical change by the identification of the activating mutations and the subsequent introduction of molecular treatments, mainly targeted to the signaling pathways generated by KIT and PDGFRA.
Most of the treatment experience in tyrosine kinase inhibitors (TKIs) is based on imatinib mesylate, where the results of long term and large simple sized studies show significant increases of the progression free survival and on the overall survival, with acceptable toxicity in most of the cases.
The therapeutic successes shown with the targeted therapy have not been evaluated in Mexico, for this reason, the present study is aimed to report the experience in a highly specialized oncology center in the systemic management of the GIST from the introduction of the TKIs.
Methods
Patients and study design
This is a retrospective, descriptive study of adult patients with the histological diagnosis of GIST, and positive immunohistochemical staining for CD117, with non-resectable or metastatic disease by extension studies and/or surgical exploration, treated with TKIs at the Oncology Hospital of National Medical Center for the Mexican Institute of Social Security.
We gathered the information of patients treated since January 1st, 2007 to March 31st, 2014.
Methods
The demographic data, histopathological characteristics, as well as the documentation of the treatment initiation dates and the clinical evolution of the patients were obtained from the electronic files.
The radiological evaluation was performed within the internal system of imaging corroborating the response through the interpretation of the control images by radiology and imaging service.
The biochemical and laboratory documentation was obtained from the internal results report.
Safety and efficacy assessment
The primary efficacy endpoint was the treatment impact on the overall survival, defined by the time from the treatment initiation until the death of the patient. The secondary endpoints were the response rates, defined in accordance with the RECIST criteria, as well as the duration of response, and additionally, the time to the recurrence in the patients who submitted to a radical primary management.
The safety and tolerability assessment was documented for all patients treated with TKIs. The adverse events were classified based on the Common Toxicity Criteria of the National Institutes of Cancer.
Statistical analysis
The efficacy analyses were performed in patients who received at least 1 dose of the TKI. The population analyses were evaluated through descriptive statistics. The response rates were established via Pearson limits with a confidence interval of 95%. The time analyses were carried out using the Kaplan-Meier and Log-rank methods. The analysis of the information was done using the software SPSS v22.0.
Results
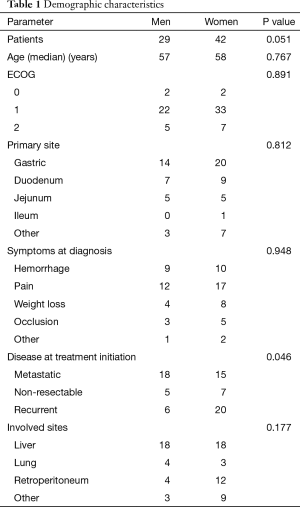
For the period evaluated, 71 GIST cases were documented, out of which 29 (40.8%) were men and 42 (59.2%) women. The overall median age was 59 years. The median age adjusted by gender showed a median of 57 for men (95% CI, 50.4–63.5) years compared with women who showed a median age of 58 (95% CI, 53–62) years, P=0.767.
At the moment of the diagnosis the performance status was distributed as follows: 5.6% had ECOG 0, 77.5% ECOG 1, and 16.9% ECOG 2 (P<0.001).
For the patients who were symptomatic at the diagnosis, the main symptoms were: upper gastrointestinal bleeding in 26%, abdominal pain 40.8%, weight loss 16.9%, bowel occlusion in 11.3%, and other symptoms in 4.2% (P=0.06)
The stages of the disease at the diagnosis were: metastatic in 46.5%, non-resectable in 16.9%, and recurrent in 36.6% (P=0.03). In the case of advanced disease, the main affected sites were: liver in 50.7%, lung in 9.9%, retroperitoneum in 22.5%, and other sites in 16.9% (P=0.04). The demographic parameters adjusted by gender are presented in Table 1.
Full table
All the patients started with systemic management with imatinib a doses de 400 mg/d with a median interval of 2 months (95% CI, 0.3–33.0).
Within the assessment of the response to the primary treatment, the stabilization of the disease was documented in 29 cases, followed by a partial response in 13, and complete response in 7 cases. Twenty two patients experienced progression with the initial management, showing a control disease rate of 49% (P<0.001) (Table 2).
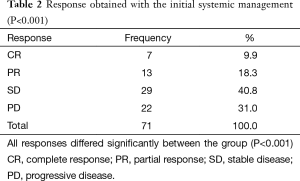
Full table
Progression free survival
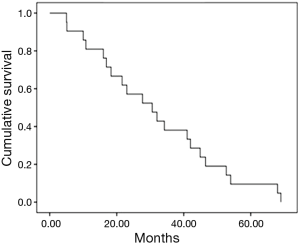
In general, the median progression free survival was 30.6 months (95% CI, 17.1–44.0) (Figure 1). Depending on the type of disease at the diagnosis, the median time to progression was 16.9 months for the metastatic disease (95% CI, 13.42–50.77), in the case of non-resectable disease the median time to progression was 23 months (95% CI, 7.1–35.31), while in the case of relapse of the disease the median time to progression was 30.6 months (95% CI, 20.5–40.6).
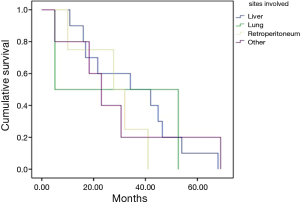
According with the extraintestinal site affected, the median progression free survivals in the study were: hepatic involvement, 34.2 months; pulmonary involvement, 5.1 months; retroperitoneal, 27.7 months; and other sites, 30.6 months (Figure 2).
Overall survival
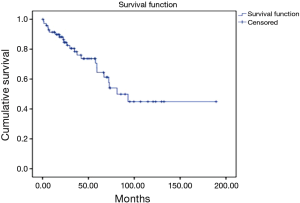
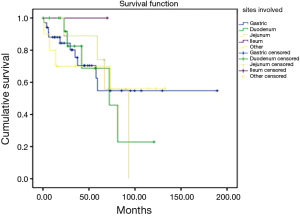
During the follow up, there were 23 deaths, showing a median estimated survival of 81.3 months (95% CI, 53.5–109) (Figure 3). A significant difference in the overall survival according with the affected site was observed, showing a median survival of 45 months for the patients with liver involvement, 25.7 months with pulmonary involvement, 36.2 months for the patients with retroperitoneal activity, and 37 months for those who presented activity in other sites (P=0.04) (Figure 4).
Toxicity
In relation to the hematologic toxicity, no statistical difference was observed in the blood counts for leukocytes, neutrophils, hemoglobin and platelets, independently of the evaluated cycle. Biochemically, there were no significant modifications observed in the follow up during the treatment (Table 3).
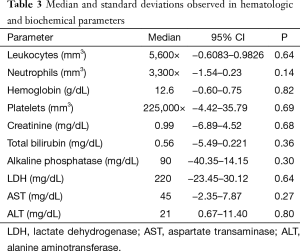
Full table
The clinical toxicity did not show relation with the administered doses, ruling out the secondary toxicity due to the cumulative doses. The observed tolerance was satisfactory and manageable in most cases, it is worth mentioning that there were 3 drug related deaths; one case due to fluid retention secondary to renal failure, and two cases in which they reported death out of the hospital, but related to infectious complications (Table 4).
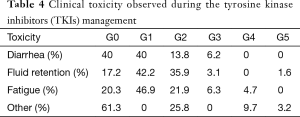
Full table
According with the clinical evaluation, the status of the patients showed a statistically significant difference in favor of the patients alive with disease, followed by death patients; followed by the group of patients with complete response and a minority group represented by patients lost to follow up (P=0.03) (Table 5).

Full table
In relation with the last treatment administered to the patient, we can observe the management continuity based on imatinib 400 mg/d, represented in 72% of the cases (P=0.002), while the dose of 800 mg/d was administered to 14.1%, sunitinib in 9.8%, and other drugs 3.3%.
Discussion
The treatment of the gastrointestinal tumors has represented a dramatic evolution in the concept of the management of the metastatic, recurrent and/or non-resectable disease, with a transition from the cytotoxic therapy which offered modest response rates, to the initiation of the molecular targeted therapy for the inhibition of the enzymatic function of the BCR-ABL oncoprotein, presented in the studies by Druker and colleagues (4). Nowadays, the targeted therapies have demonstrated activity in vivo through the immune modulation, potentializing the antitumoral response through an increase of the T cells activity (5).
The demographic data obtained from our population showed a trend towards females, which contrasts with the published data, where there is a men:women ratio of 1–1.5:1, in comparison, we observed in our study an turn around in the mentioned ratio. In relation with the age group, the median reported in European series is 66–69 years (6,7), and by the SEER (63 years) (8). The results show a median ten years lower for the age at the diagnosis, a very relevant figure for the cases in the Mexican population.
The relation of the sites affected with advanced disease correlates with data reported from international series, with a preponderance of hepatic activity, followed by lung, and retroperitoneal involvement in third place.
The clinical information comes from the results shown by Joensuu and colleagues, where a prolonged clinical response was documented with times surpassing 24 months in patients treated with imatinib (9). The tumor response rates in GIST patients, treated with imatinib reported in the trials by van Oosterom, Demetri, Verweij and Blanke (9-14) show as main treatment response stable disease with ranges of 45% to 56%; with complete response rates of 2% to 5%, and partial response of 20%, which translate into a clinical benefit of 70% to 90%, independently of the dose of 400, 600 or 800 mg/d. The results obtained in our site with imatinib doses of 400 mg/d show a complete response rate of 10%, partial response of 18%, and stable disease in 40% of the cases, reflecting a 70% clinical benefit, with similar rates to those reported in the series previously cited.
With respect to the overall survival, the study with the longest follow up (71 months), published by Blanke and colleagues (15) reports a median of 57 months, independently of the imatinib dose administered, in comparison, the series presented show a superior survival, with an estimated median of 81 months. It is worth mentioning that the difference may be influenced by the modifications made to the doses and regimens in case of progression, unlike the above mentioned study where the drug response is specifically evaluated to a pre-established dose. Additionally, it is necessary to consider the tumor biology that conditions a higher sensitivity to the treatment with TKIs, this warrants the need for the molecular evaluation of the tumors in patients treated at our hospital.
Unlike the existing information, in our study, we performed a subanalysis of the patients who died from the disease in correlation with the sites of primary involvement. It was possible to show a decrease in the survival in the cases with pulmonary involvement versus the hepatic, retroperitoneal, and other sites activity (P=0.04), establishing the pulmonary metastasis as a factor of poor prognosis in advanced GIST.
Conclusions
This study represents one of the most important reviews of the GIST in Mexico. It documents different and relevant conditions in the diagnosis and response to treatment, to those documented in international series. A higher tumor response is observed with the subsequent impact on the overall survival. This study sets the bases to initiate studies at the molecular level aimed to determine biological differences that justify the clinical behavior, characteristics of our group cases.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The trial was approved by the institutional review board or ethics committee at each center and complied with Good Clinical Practice guidelines, the Declaration of Helsinki, and local laws. All patients provided written informed consent.
References
- Rubin BP, Fletcher JA, Fletcher CD. Molecular Insights into the Histogenesis and Pathogenesis of Gastrointestinal Stromal Tumors. Int J Surg Pathol 2000;8:5-10. [Crossref] [PubMed]
- Miettinen M, Lasota J. Gastrointestinal stromal tumors--definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch 2001;438:1-12. [Crossref] [PubMed]
- Demetri GD, Elias AD. Results of single-agent and combination chemotherapy for advanced soft tissue sarcomas. Implications for decision making in the clinic. Hematol Oncol Clin North Am 1995;9:765-85. [PubMed]
- Druker BJ, Tamura S, Buchdunger E, et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med 1996;2:561-6. [Crossref] [PubMed]
- Balachandran VP, Cavnar MJ, Zeng S, et al. Imatinib potentiates antitumor T cell responses in gastrointestinal stromal tumor through the inhibition of Ido. Nat Med 2011;17:1094-100. [Crossref] [PubMed]
- Nilsson B, Bümming P, Meis-Kindblom JM, et al. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era--a population-based study in western Sweden. Cancer 2005;103:821-9. [Crossref] [PubMed]
- Tryggvason G, Gíslason HG, Magnússon MK, et al. Gastrointestinal stromal tumors in Iceland, 1990-2003: the icelandic GIST study, a population-based incidence and pathologic risk stratification study. Int J Cancer 2005;117:289-93. [Crossref] [PubMed]
- Tran T, Davila JA, El-Serag HB. The epidemiology of malignant gastrointestinal stromal tumors: an analysis of 1,458 cases from 1992 to 2000. Am J Gastroenterol 2005;100:162-8. [Crossref] [PubMed]
- Joensuu H, Roberts PJ, Sarlomo-Rikala M, et al. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med 2001;344:1052-6. [Crossref] [PubMed]
- van Oosterom AT, Judson I, Verweij J, et al. Safety and efficacy of imatinib (STI571) in metastatic gastrointestinal stromal tumours: a phase I study. Lancet 2001;358:1421-3. [Crossref] [PubMed]
- Verweij J, van Oosterom A, Blay JY, et al. Imatinib mesylate (STI-571 Glivec, Gleevec) is an active agent for gastrointestinal stromal tumours, but does not yield responses in other soft-tissue sarcomas that are unselected for a molecular target. Results from an EORTC Soft Tissue and Bone Sarcoma Group phase II study. Eur J Cancer 2003;39:2006-11. [Crossref] [PubMed]
- Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 2002;347:472-80. [Crossref] [PubMed]
- Blanke CD, Rankin C, Demetri GD, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol 2008;26:626-32. [Crossref] [PubMed]
- Verweij J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet 2004;364:1127-34. [Crossref] [PubMed]
- Blanke CD, Demetri GD, von Mehren M, et al. Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol 2008;26:620-5. [Crossref] [PubMed]