A comparison between 5-fluorouracil/mitomycin and capecitabine/mitomycin in combination with radiation for anal cancer
Introduction
Carcinoma of the anal canal is a relatively rare malignancy, accounting for approximately 2.4% of all gastrointestinal cancers (1). Although uncommon, its incidence has been on the rise for the past decades, possibly due to the increased sexual transmission of human papilloma virus (HPV) (2-6). Historically, treatment of anal cancer consisted of abdominoperineal resection (APR) with inguinal lymph node dissection, which required removal of the anorectum and creation of a permanent colostomy (7-9).
More recently, combined modality therapy with radiation (RT) and concurrent chemotherapy has emerged as the preferred method of treatment for squamous cell cancer (SCC) of the anal canal, which has significantly reduced the rates of locoregional recurrence and obviated the need for a permanent colostomy in most cases (10,11). Mitomycin and 5-fluorouracil (5-FU) remain the standard chemotherapy regimen for combination with RT at most centers worldwide. The original regimen widely known as the “Wayne State protocol” uses infusional 5-FU 1,000 mg/m2 on days 1 to 4 and 29 to 32 in combination with mitomycin 10 to 15 mg/m2 on day 1 concurrent with RT (10). However, a modified version has been in use since the RTOG 98–11 phase III trial (11), consisting of the same 5-FU doses combined with mitomycin 10 mg/m2 on days 1 and 29. Four-year colostomy-free survival (CFS) and disease-free survival (DFS) were 71% and 73%, respectively.
Capecitabine is an oral fluoropyrimidine shown to be equivalent to infusional 5-FU when given concurrently with RT in the neoadjuvant treatment of rectal adenocarcinoma (12-14). Given important advantages in terms of convenience and a decrease in the use of hospital resources (such as intravenous chemotherapy administration and central catheter insertion) for the substitution of infusional 5-FU for capecitabine, many centers have been recently adopting capecitabine plus mitomycin concurrent with RT. However, no randomized trials in anal canal cancer have so far compared 5-FU and capecitabine. A non-randomized phase II trial of 31 patients has explored the use of capecitabine, mitomycin and RT in anal cancer patients. RT comprised 50.4 Gy in 28 fractions of 1.8 Gy with mitomycin 12 mg/m2 on day 1 and capecitabine 825 mg/m2 twice daily on each RT treatment day. Complete clinical response was observed in 77% 4 weeks after completion of therapy and there were only 3 locoregional recurrences at a median follow-up of 14 months (15).
The aim of our study was to compare the outcomes of patients treated with chemoradiation with 5-fluorouracil/mitomycin (FM) versus capecitabine/mitomycin (CM).
Methods
Characteristics of the study setting and study population
British Columbia (BC) is a Canadian province with a population of 4.4 million. The BC Cancer Agency (BCCA) is responsible for funding all systemic cancer therapy and is the sole provider of radiotherapy in BC. All patients in the province who require radiation therapy for a diagnosis of SCC or cloacogenic carcinoma of the anal canal are referred to 1 of 5 BCCA centers for consultation and treatment delivery.
Initially chemoradiation with FM was the standard therapy for patients with non-metastatic anal cancer at our institution. Treatment recommendation consisted of radiation in combination with infusional 5-FU 1,000 mg/m2 on days 1 to 4 and 29 to 32 and mitomycin 10 mg/m2 (maximum dose: 20 mg) on days 1 and 29. In February 2010, a standardized protocol of chemoradiation with CM was introduced and posted on the BCCA website, offering the substitution of capecitabine for infusional 5-FU (16). It includes the same radiation schedule. Capecitabine is delivered twice a day at a dose of 825 mg/m2 on days that radiotherapy is administered (days 1–5, 8–12, 15–19, 22–26, 29–33, 36–40), to a total daily dose of 1,650 mg/m2. Until 2012 mitomycin was administered on day one only at a dose of 12 mg/m2 (maximum dose: 20 mg), but the most recent protocol allows mitomycin administration at a dose of 10 mg/m2 on days 1 and 29.
At our institution radiation therapy for stage I–III anal cancer typically includes a minimum dose of 45 Gy in 25 fractions of 1.8 Gy over 5 weeks to the primary cancer with supervoltage radiation (photon energy of >6 mv) using anteroposterior-posteroanterior (AP-PA) or multifield techniques. For patients with T3, T4, node-positive disease or patients with T2 residual disease after 45 Gy, the intent is to deliver an additional boost of 10 to 14 Gy in 2 Gy fractions (total dose of 55–59 Gy in 30–32 fractions over 5.5–6.5 weeks).
Records of patients who received curative-intent radiation with either FM or CM for newly diagnosed stage I–III anal cancer between 1998 and 2013 at the BCCA were reviewed. Initially 637 patients who received chemoradiation for anal cancer were identified through the pharmacy database. Sixty-four were excluded due to either prior APR or evidence or metastatic disease before embarking on chemoradiation. Another 23 patients were excluded due to the use of cisplatin instead of mitomycin. Finally, 250 patients were excluded due to suboptimal radiation doses. The final cohort consisted of 300 patients. The local institutional review board approved this study.
Eligibility criteria for chemoradiation with either FM or CM at the BCCA include a diagnosis of stage I–III squamous cell or cloacogenic carcinoma of the anal canal and Eastern Cooperative Oncology Group (ECOG) performance status (PS) of less than or equal to 2. Patients also need to have an adequate marrow reserve (neutrophils greater than or equal to 1.5×109/L, platelets greater than 100×109/L), with adequate renal (creatinine less than or equal to 1.5× ULN) and liver function (bilirubin less than or equal to 26 µmol/L; AST/Alkaline Phosphatase less than or equal to 5× ULN).
Statistical analysis
Baseline characteristics were summarized with descriptive statistics. Variations in baseline parameters across the different groups (FM and CM) were evaluated using the Chi-square test for categorical variables and the F test for continuous variables.
The primary endpoint of DFS was calculated from the date of the first treatment to the date of disease recurrence (metastasis or local recurrence after prior complete clinical response), disease persistence (histologically documented persistent cancer in the treated radiation field), or death from any cause, whichever occurred first. Anal cancer specific survival (ACSS) was measured from the date of the first treatment to the date of death by anal cancer. Deaths from other causes were censured. The 2-year DFS and 2-year ACSS were estimated for the different groups using the Kaplan-Meier method. Differences in survival outcomes were assessed with the log-rank test.
Multivariate Cox proportional hazards regression models were subsequently fitted to evaluate the relationship of chemotherapy regimen (CM vs. FM) on DFS and ACSS. The multivariate model was constructed incorporating the significant prognostic factors found in the univariate log-rank test. They were expressed as hazard ratios (HR) and 95% confidence intervals (95% CI) for relapse. All tests were two-sided where a P value of <0.05 was considered statistically significant. SPSS (version 14.0) was used to conduct all of the statistical analyses.
Results
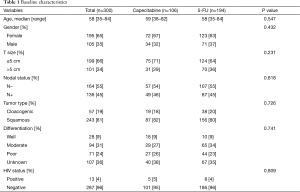
A total of 300 patients (65% females) were included in the analysis. Median age was 58 years. Median tumor size was 4 cm (66% ≤5 cm and 34% >5 cm). The majority (54.7%) had clinical negative lymph nodes based on either imaging or physical examination. Histology included basaloid/cloacogenic carcinoma in 19% and SCC in 81%. In terms of tumor differentiation, 9% were well, 31% moderately, 24% poorly differentiated and 36% had missing information. Only 13 patients (4%) were HIV positive. One-hundred and six patients (35.3%) received chemoradiation with CM while 194 (64.7%) were treated with FM. Baseline patient and tumor characteristics were well balanced between the two groups (Table 1). More patients received 2 cycles of mitomycin in the FM group (69% vs. 40%, P<0.001). There was no difference in radiation completion among the groups (96 for FM vs. 94% for CM, P=0.546) (Table 2).
Full table

Full table
Median follow-up was 43.9 months (23.8 months for CM vs. 60.2 months for FM, P<0.001). By the time of the analysis, 63 patients had died (14 in the CM and 49 in the FM group) and 64 experienced either disease persistence or recurrence. Anal cancer death accounted for the majority of death cases (83.6%). For those patients with persistent disease, median time to biopsy-proven diagnosis was 5.2 months (95% CI, 4.1–6.3). Among the 64 patients with a disease event, only 9.3% were diagnosed after 2 years.
Univariate analysis
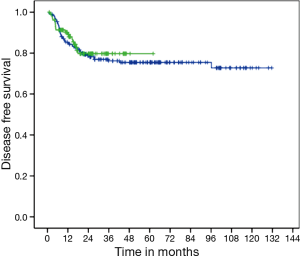
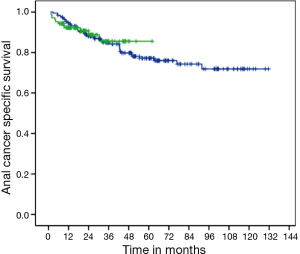
There was no difference in DFS between CM and FM (medians not reached, P=0.663; HR =1.13; 95% CI, 0.65–1.96) (Figure 1). Two-years DFS was 79.7% for CM (95% CI, 71.1–88.3%) and 78.8% for FM (95% CI, 73–84.6%). Anal cancer-specific survival (ACSS) was also similar between groups (medians not reached, P=0.839) (Figure 2). Two-years ACSS was 88.7% for CM (95% CI, 81.8–95.5%) and 87.5% for FM (95% CI, 82.8–92.2%). There was no difference in failure colostomy-free survival (medians not reached, P=0.336; HR =0.66; 95% CI, 0.28–1.54). APR-free survival was also similar between CM and FM (medians not reached, P=0.520; HR =0.75; 95% CI, 0.31–1.79).
Patients with HIV+ had shorter DFS (P=0.007; HR =3.02; 95% CI, 1.30–7.01) and ACSS (P=0.008; HR =3.29; 95% CI, 1.30–8.31). Tumors larger than 5 cm also had shorter DFS (P<0.001; HR =3.64; 95% CI, 2.20–6.00) and ACSS (P<0.001; HR =4.69; 95% CI, 2.61–8.40). Clinical node positivity conferred worse DFS (P<0.001; HR =4.30; 95% CI, 2.44–7.59) and ACSS (P<0.001; HR =4.61; 95% CI, 2.41–8.82). Males had worse DFS (P=0.003; HR =2.08; 95% CI, 1.27–3.41) and a trend towards worse ACSS (P=0.086; HR =1.62; 95% CI, 0.93–2.81). There was a trend towards better DFS for patients ≥60 years of age (P=0.061; HR =0.58; 95% CI, 0.37–1.02), but no difference in ACSS (P=0.139; HR =0.65; 95% CI, 0.36–1.14).
There was no difference in DFS and ACSS between cloacogenic/basaloid and SCC histology (P=0.644 and 0.340, respectively) or between well, moderately, and poorly differentiated tumors (P=0.177 and 0.089, respectively). Likewise, DFS and ACSS were similar among patients who received 1 or 2 cycles of mitomycin (P=0.499 and 0.943, respectively).
Multivariate analysis
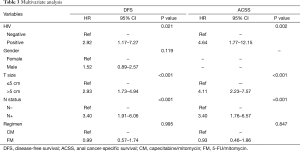
On multivariate analysis, only HIV status, clinical T size (≤5 vs. >5 cm), and clinical N status (negative vs. positive) remained as significant prognostic factors for DFS. The chemotherapy regimen had no impact on DFS (P=0.995; HR =0.99; 95% CI, 0.57–1.74). Similar results were found for ACSS, in which only HIV status, T size, and N status were significant prognostic factors. The chemotherapy regimen also had no impact on ACSS (P=0.847; HR =0.93; 95% CI, 0.46–1.86). Table 3 shows the results of the multivariate analysis for both DFS and ACSS.
Full table
Discussion
Our results support the substitution of capecitabine for infusional 5-FU in combination with mitomycin and pelvic radiation to a total dose of 50.4 Gy in 28 daily fractions over 5.5 weeks for curative-intent treatment of anal cancer. Both DFS and ACSS were similar between CM and FM. To the best of our knowledge, this is the first study to compare CM and FM concomitant with radiation in the treatment of stage I–III anal cancer. So far the only data supporting CM comes from a small non-randomized phase II trial, in which 90% of the patients achieved local control at 6 months (15).
Similarly to other studies (17-20), we also demonstrated that tumor size, nodal status and HIV status are the most significant prognostic factors for patients with anal SCC. On multivariate analysis, patients with tumors >5 cm as well as those with HIV+ had almost 3 times more risk of developing a disease event and at least 4 times the risk of dying from anal cancer. Clinical node positivity conferred 3.4 times the risk of both a disease event and death from anal cancer. Although two prior studies demonstrated poor outcomes for male sex (21,22), the results of our multivariate analysis did not support gender as a prognostic factor.
The favorable outcomes achieved by capecitabine in substitution for 5-FU in the present study are in accordance with other gastrointestinal malignancies, where capecitabine has been studied more extensively. A recent meta-analysis, based on individual patient data from six randomized non-inferiority trials, compared the effects of single-agent capecitabine or capecitabine-containing chemotherapy versus matched 5-FU-based regimens in terms of overall survival (OS) in patients with stage III colon, metastatic colorectal or advanced gastric cancer (23). The unadjusted HR for OS for capecitabine-containing chemotherapy versus 5-FU-containing chemotherapy was 0.94 (95% CI, 0.89–1.00, P=0.0489), showing that capecitabine and 5-FU can be used interchangeably in these gastrointestinal patient populations (23).
The choice of capecitabine over 5-FU is therefore primarily based on ease of administration in addition to differences in toxicity. In general, there is less mucositis, nausea and neutropenia with capecitabine-containing regimens, with the trade-off of more hand-foot syndrome reactions (24). Aside from different adverse events, using capecitabine in combination therapy avoids the use of long-term indwelling catheters, infusion pumps, and their potential complications, allied to reduced frequency of clinic visits (25,26). Cost comparisons of capecitabine monotherapy versus 5-FU/leucovorin show that the higher drug cost per se of capecitabine is partially or completely offset by costs associated with treating toxicity and the higher administration costs of intravenous 5-FU/leucovorin in both the metastatic (27-30) and adjuvant (30-34) settings.
Although this study included a large population-based series reflecting the real-world practice, it does have several limitations including the retrospective nature of the data collection and the lack of toxicity report. In parallel, patients who received CM had significantly shorter median follow-up time since capecitabine was only approved for use concomitant with radiation and mitomycin in 2010 at our institution. Nonetheless, only 9.3% of patients who experience disease persistence or recurrence were diagnosed after 24 months. In addition, we have not collected data on toxicity or rates of chemotherapy dose reduction. Differently than in clinical trials, the precise surveillance strategy after the completion of chemoradiation was not captured in our study. Typically, after the completion of chemoradiation, a digital examination is performed within 6 to 8 weeks at our institution. Thereafter, patients are followed up every 3 months for 2 years with rectal examination, anoscopy and examination of inguinal lymph nodes, and then every 6 months for 3 additional years. For tumors with extensive rectal involvement and/or pelvic nodal involvement, CT, magnetic resonance imaging (MRI) or positron emission tomography (PET)/CT may be considered as an adjunct to clinical examination. However, whether patients were being followed up according to those guidelines was not confirmed.
In conclusion, CM and FM concomitant with radiotherapy achieved similar DFS and ACSS in our study. Substitution of capecitabine for infusional 5-FU may therefore be a reasonable option for patients and physicians who prefer to avoid the inconvenience and potential complications of a central infusional device. While no randomized trials are under way comparing CM and FM, our retrospective study supports the use of CM concomitant with radiation therapy for patients with anal cancer.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The local institutional review board approved this study.
References
- Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29. [Crossref] [PubMed]
- Bilimoria KY, Bentrem DJ, Rock CE. Outcomes and prognostic factors for squamous-cell carcinoma of the anal canal: analysis of patients from the National Cancer Data Base. Dis Colon Rectum 2009;52:624-31. [Crossref] [PubMed]
- Ryan DP, Mayer RJ. Anal carcinoma: histology, staging, epidemiology, treatment. Curr Opin Oncol 2000;12:345-52. [Crossref] [PubMed]
- Moscicki AB, Schiffman M, Burchell A, et al. Updating the natural history of human papillomavirus and anogenital cancers. Vaccine 2012;30:F24-33. [Crossref] [PubMed]
- Serup-Hansen E, Linnemann D, Skovrider-Ruminski W, et al. Human papillomavirus genotyping and p16 expression as prognostic factors for patients with American Joint Committee on Cancer stages I to III carcinoma of the anal canal. J Clin Oncol 2014;32:1812-7. [Crossref] [PubMed]
- Johnson LG, Madeleine MM, Newcomer LM, et al. Anal cancer incidence and survival: the surveillance, epidemiology, and end results experience, 1973-2000. Cancer 2004;101:281-8. [Crossref] [PubMed]
- Schraut WH, Wang CH, Dawson PJ, et al. Depth of invasion, location, and size of cancer of the anus dictate operative treatment. Cancer 1983;51:1291-6. [Crossref] [PubMed]
- Singh R, Nime F, Mittelman A. Malignant epithelial tumors of the anal canal. Cancer 1981;48:411-5. [Crossref] [PubMed]
- Pintor MP, Northover JM, Nicholls RJ. Squamous cell carcinoma of the anus at one hospital from 1948 to 1984. Br J Surg 1989;76:806-10. [Crossref] [PubMed]
- Nigro ND, Vaitkevicius VK, Considine B Jr. Combined therapy for cancer of the anal canal: a preliminary report. Dis Colon Rectum 1974;17:354-6. [Crossref] [PubMed]
- Flam M, John M, Pajak TF, Petrelli N, et al. Role of mitomycin in combination with fluorouracil and radiotherapy, and of salvage chemoradiation in the definitive nonsurgical treatment of epidermoid carcinoma of the anal canal: results of a phase III randomized intergroup study. J Clin Oncol 1996;14:2527-39. [PubMed]
- Dunst J, Reese T, Sutter T, et al. Phase I trial evaluating the concurrent combination of radiotherapy and capecitabine in rectal cancer. J Clin Oncol 2002;20:3983-91. [Crossref] [PubMed]
- De Paoli A, Chiara S, Luppi G, et al. Capecitabine in combination with preoperative radiation therapy in locally advanced, resectable, rectal cancer: a multicentric phase II study. Ann Oncol 2006;17:246-51. [Crossref] [PubMed]
- Hofheinz RD, Wenz F, Post S, et al. Chemoradiotherapy with capecitabine versus fluorouracil for locally advanced rectal cancer: a randomised, multicentre, non-inferiority, phase 3 trial. Lancet Oncol 2012;13:579-88. [Crossref] [PubMed]
- Glynne-Jones R, Meadows H, Wan S, et al. EXTRA--a multicenter phase II study of chemoradiation using a 5 day per week oral regimen of capecitabine and intravenous mitomycin C in anal cancer. Int J Radiat Oncol Biol Phys 2008;72:119-26. [Crossref] [PubMed]
- BCCA protocol summary for curative combined modality therapy for carcinoma of the Anal canal using mitomycin, capecitabine and radiation therapy. Available online: . Accessed May 30, 2013.http://www.bccancer.bc.ca/chemotherapy-protocols-site/Documents/Gastrointestinal/GICART_Protocol_1Nov2015.pdf
- Pintor MP, Northover JM, Nicholls RJ. Squamous cell carcinoma of the anus at one hospital from 1948 to 1984. Br J Surg 1989;76:806-10. [Crossref] [PubMed]
- Touboul E, Schlienger M, Buffat L, et al. Epidermoid carcinoma of the anal canal. Results of curative-intent radiation therapy in a series of 270 patients. Cancer 1994;73:1569-79. [Crossref] [PubMed]
- Klas JV, Rothenberger DA, Wong WD, et al. Malignant tumors of the anal canal: the spectrum of disease, treatment, and outcomes. Cancer 1999;85:1686-93. [Crossref] [PubMed]
- Oehler-Jänne C, Huguet F, Provencher S, et al. HIV-specific differences in outcome of squamous cell carcinoma of the anal canal: a multicentric cohort study of HIV-positive patients receiving highly active antiretroviral therapy. J Clin Oncol 2008;26:2550-7. [Crossref] [PubMed]
- Ajani JA, Winter KA, Gunderson LL, et al. Prognostic factors derived from a prospective database dictate clinical biology of anal cancer: the intergroup trial (RTOG 98-11). Cancer 2010;116:4007-13. [Crossref] [PubMed]
- Glynne-Jones R, Sebag-Montefiore D, Adams R, et al. Prognostic factors for recurrence and survival in anal cancer: generating hypotheses from the mature outcomes of the first United Kingdom Coordinating Committee on Cancer Research Anal Cancer Trial (ACTI). Cancer 2013;119:748-55. [Crossref] [PubMed]
- Cassidy J, Saltz L, Twelves C, et al. Efficacy of capecitabine versus 5-fluorouracil in colorectal and gastric cancers: a meta-analysis of individual data from 6171 patients. Ann Oncol 2011;22:2604-9. [Crossref] [PubMed]
- Hoff PM, Ansari R, Batist G, et al. Comparison of oral capecitabine versus intravenous fluorouracil plus leucovorin as first-line treatment in 605 patients with metastatic colorectal cancer: results of a randomized phase III study. J Clin Oncol 2001;19:2282-92. [PubMed]
- Twelves CJ. Xeloda in Adjuvant Colon Cancer Therapy (X-ACT) trial: overview of efficacy, safety, and cost-effectiveness. Clin Colorectal Cancer 2006;6:278-287. [Crossref] [PubMed]
- Hebbar M, Bennouna J, Boige V, et al. Safety and quality of life (QoL) findings from a randomized phase III study of capecitabine (X) + oxaliplatin (O) (XELOX) vs. infusional 5-FU/LV + O (FOLFOX-6) in metastatic colorectal cancer (MCRC). J Clin Oncol 2007;25:4099. (Meeting Abstracts).
- Ward S, Kaltenthaler E, Cowan J, et al. Clinical and cost-effectiveness of capecitabine and tegafur with uracil for the treatment of metastatic colorectal cancer: systematic review and economic evaluation. Health Technol Assess 2003;7:1-93. [Crossref] [PubMed]
- Horgan AM, Knox JJ, Liu G, et al. Capecitabine or infusional 5-fluorouracil for gastroesophageal cancer: a cost-consequence analysis. Curr Oncol 2011;18:e64-70. [Crossref] [PubMed]
- Wiklund TA, Pekurinen M. Pharmacoeconomic comparison of capecitabine vs. intravenous 5-FU/leucovorin (Mayo protocol) in colorectal cancer (CRC) in Finland T. Proc Am Soc Clin Oncol 2003;22:530.
- Jansman FG, Postma MJ, van Hartskamp D, et al. Cost-benefit analysis of capecitabine versus 5-fluorouracil/leucovorin in the treatment of colorectal cancer in the Netherlands. Clin Ther 2004;26:579-89. [Crossref] [PubMed]
- Cassidy J, Douillard JY, Twelves C, et al. Pharmacoeconomic analysis of adjuvant oral capecitabine vs intravenous 5-FU/LV in Dukes' C colon cancer: the X-ACT trial. Br J Cancer 2006;94:1122-9. [Crossref] [PubMed]
- Virik K, Skedgel C, Younis T. Economic evaluation of adjuvant chemotherapy in stage III (SIII) colon cancer: capecitabine versus 5FU/LV. J Clin Oncol 2006;24:6064. (Meeting Abstracts).
- Di Costanzo F, Ravasio R, Sobrero A, et al. Capecitabine versus bolus fluorouracil plus leucovorin (folinic acid) as adjuvant chemotherapy for patients with Dukes' C colon cancer: economic evaluation in an Italian NHS setting. Clin Drug Investig 2008;28:645-55. [Crossref] [PubMed]
- Chu E, Schulman KL, Zelt S, et al. Costs associated with complications are lower with capecitabine than with 5-fluorouracil in patients with colorectal cancer. Cancer 2009;115:1412-23. [Crossref] [PubMed]