Neoadjuvant imatinib: longer the better, need to modify risk stratification for adjuvant imatinib
Introduction
Gastrointestinal stromal tumor (GIST) accounts for 1% of primary GI tumors. GISTs may arise from any part of GI tract and clinical presentation correlates with site, size and rapidity of growth (1,2). While surgical resection has been the main modality of treatment (3), many cases are locally advanced and unresectable at presentation. Besides, post curative resections, approximately 40% cases will develop recurrences in the form of local and/or distant metastasis (4,5). Imatinib mesylate (IM), the multi-kinase targeted therapy, for a duration of 3 years is now the standard adjuvant therapy after surgical resection of non-metastatic GISTs with significant risk of recurrence (5-7). In locally advanced or unresectable GISTs, use of neoadjuvant IM helps in decreasing the extent of resection (also by avoiding multivisceral resections) and surgical morbidity by downsizing the tumor, and reducing the intraoperative spillage of tumor cells (8-10). There is growing evidence for neoadjuvant IM therapy in terms of disease free survival (DFS) and overall survival (OS), with major evidence of benefit shown in the EORTC-STBSG retrospective analysis (11-15).
The purpose of this study was to evaluate the demographic profile, presentation and outcomes of 112 patients with GIST who underwent surgery at our institution and were potential candidates for either neoadjuvant or adjuvant IM over a period of 6 years. We also attempted to identify potential prognostic factors with respect to outcomes and placed special emphasis on patients receiving neoadjuvant IM.
Methods
A retrospective analysis of prospectively maintained database of all GIST patients who underwent surgery and presented between January 2009 to March 2015 at Department of GI & Hepatopancreaticobiliary Oncology, Tata Memorial Hospital, Mumbai, was performed. Clinical and radiological data were recorded from patient files and electronic medical records. Patients presenting in the above study period were subdivided on the basis of clinical presentation and treatment received into localized operable, locally advanced, operated elsewhere on adjuvant treatment and incidental GISTs. Locally advanced GISTs were defined by the size, need for multivisceral resections, anatomic proximity with major vessels and risk of intraoperative tumor spillage. Patients operated elsewhere on adjuvant treatment were not included in this analysis. Incidental GISTs were recorded when the patient was operated for some different malignancy and final histopathology report shows additional incidental GIST. Demographic profile, clinic-pathological presentation and radiological characteristics including age, sex, chief complaints, site of primary, size, histology with IHC, and duration of IM therapy were recorded for all 112 patients.
All locally advanced GIST patients received neoadjuvant imatinib therapy after discussion in tumor board meetings & joint clinics. Response to neoadjuvant treatment was recorded as per RECIST criteria and evaluated with CT scans every 2–3 months interval. Response was categorized into four categories as complete response (CR), partial response (PR), and stable disease (SD). Pathological evaluation in terms of confirmation of diagnosis, tumor characteristics and response to neoadjuvant treatment was done by a team of expert GI pathologists. Risk stratification into very low, low, intermediate and high risk was done using revised NIH risk criteria 2008. Tumors were staged as per latest TNM staging system AJCC 2010. Adjuvant treatment was decided in joint clinics depending on the risk stratification. Follow up policy was every 3 months for first 2 years, every 6 months for next 3 years and yearly thereafter for next 5 years. Patient status, local recurrence and development of metastatic disease were recorded during follow ups at regular intervals.
Statistics
All data was entered in SPSS software version 20 and used for analysis. χ2 test and Fisher’s exact test were performed to analyze qualitative parameters. Survival outcomes in terms of DFS and OS were analyzed. DFS was calculated from the date of surgical resection to the date of clinical or radiological evidence of disease relapse or the last follow up date. OS was calculated from the date of first administration of imatinib for locally advanced GISTs or date of surgery for upfront operable GISTs until last follow up or death. All deaths from causes other than tumor related were recorded as censored for DSS calculations. Survival analysis was done using Kaplan-Meier estimates and log rank test for bivariate comparisons. Multivariate analysis was done using Cox proportional hazard analysis.
Results
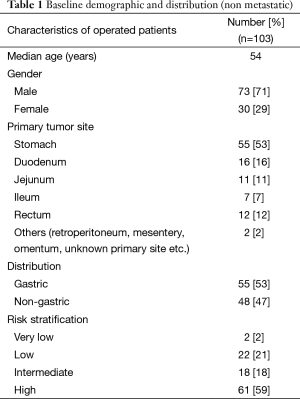
A total of 112 patients were operated during the period of analysis. The categorical distribution of operated GISTs patients includes localized operable 27 (24%), locally advanced at presentation 76 (68%) and potentially resectable but metastatic at presentation n=9 (8%) (Table 1).
Full table
Demographic & clinico-pathological characteristics: localized operable and locally advanced
Baseline data for the non metastatic patients showed a median age of 54 years (16–82 years). A total of 71% were males and 29% were females with male to female ratio of 2.4:1 (Table 1).
The site wise distribution of primary tumors included stomach 55 (53%), duodenum 16 (16%), rectum 12 (12%), jejunum 11 (11%), ileum 7 (7%), and others including retro peritoneum, mesentery, and unknown primary site 2 (2%) (Table 1).
Histopathology/stage & risk stratification
The median tumor size was 7.25 cm (range from 2–30 cm). The median mitotic index was 5 per 50 hpf (range from 0–300 per 50 hpf). Lymph node metastasis was found in 1 patient. The final histopathology report shows no residual viable tumor in 12 cases. Risk stratification as per the revised NIH criteria 2008 classified the cohort of GISTs into very low risk (2%), low risk (21%), intermediate risk (18%) and high risk (59%) (Table 1).
Responses and IM therapy (Table 2)
Full table
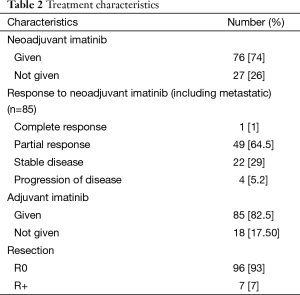
Twenty-seven patients underwent upfront surgery for primary site. A total of 76 out of 123 (61%) locally advanced GIST patients became operable after neoadjuvant IM while the remaining 47 patients are still on therapy and under potential evaluation for surgery at a later date. The median duration of neoadjuvant therapy was 5 months (range from 1–98 months). The objective changes in tumor size preimatinib to postimatinib as per RECIST criteria were reported by our GI radiologist. Out of 76 patients, 50 (64.55%) patients had PR, 1 (1%) patient had CR, 23 (29%) had SD while 4 (5.2%) patients had progression of disease while on neoadjuvant IM therapy. R0 resection was done in 96 patients (93%) of patients while 7 (7%) patients had R+ resections. The adjuvant IM therapy was given to 85 patients (82.50%). Median duration of adjuvant imatinib therapy was 21 months with range from 1–49 months. Median follow up was 30.2 months with range from 0.92–113 months.
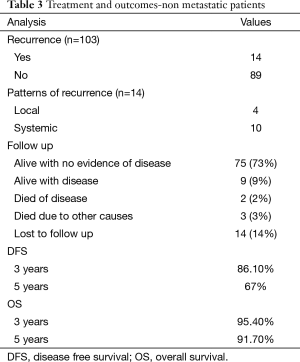
Overall, 14 patients develop recurrence during the follow up with 4 patients having local recurrence, and 10 patients developing systemic recurrence. Eleven patients developed recurrence while on adjuvant IM therapy while the other 3 patients had not received adjuvant imatinib therapy (Table 3). Median DFI of patients who developed recurrence was 20.5 months with range from 2.50–61.2 months.
Full table
Till last follow up, 75 patients are alive with no evidence of disease, 9 patients are alive with disease recurrence, and 2 patients died of the disease progression while 3 patients died of causes other than disease progression. Fourteen patients were lost to follow up of whom 11 were on adjuvant imatinib.
Characteristics of metastatic but operated patients (Table 4)
Full table
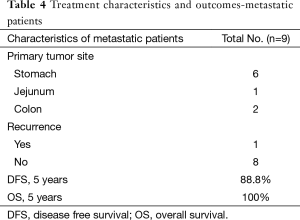
Nine patients with upfront metastatic disease had responded to IM therapy at primary & metastatic site. Cases were discussed in multidisciplinary joint clinics for surgical resection and treatment with curative intent. Four patients underwent both surgery at primary site and metastatic site while the other 5 patients underwent surgical resection of primary site as metastatic site was non FDG avid on PET CT scans. R0 resection at primary site was achievable in all patients while 1 patient had involved focal margin at metastatic site. All patients are alive with a median follow up period of 24 months with range from 4–95 months. All patients received adjuvant IM therapy with a median duration of 13 months (range from 2–54 months).
Survival analysis & prognostic factors
Survival analysis was done separately in two subgroups depending upon metastatic disease or non-metastatic disease at presentation.
Survival analysis and prognostic factors of non- metastatic patients (Table 3)
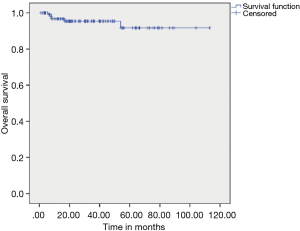
The median DFS after primary resection of GIST was not reached, and estimated 3 & 5 years DFS was 86.10% & 67% respectively. The median OS has not been reached, and estimated 3- & 5-year OS was 95.40% and 91.70% respectively (Figures 1,2).

On univariate analysis of prognostic factors affecting PFS, female patients did better than male patients (P=0.004), and patients undergoing upfront surgery followed by adjuvant IM did better than those not receiving adjuvant IM (P=0.04). However, on multivariate analysis, only adjuvant IM was a significant factor for survival (P=0.05) compared to patients receiving no adjuvant IM.
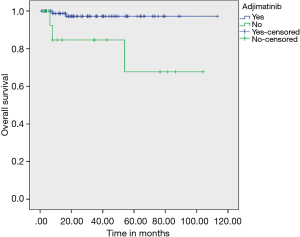
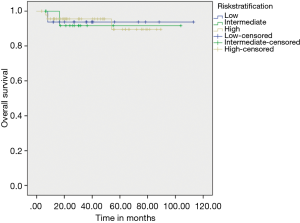
On evaluating prognostic factors affecting OS, age <50 years had better OS than age >50 years (P=0.037) and patients who received adjuvant IM had better OS (97.20% vs. 67.70%) than who did not received adjuvant IM therapy (P=0.003) (Figure 3). Adjuvant IM remained a significant factor on multivariate analysis as well (P=0.023). Risk stratification did not have an effect on outcomes (Figure 4).
Survival analysis of metastatic patients (Table 4)
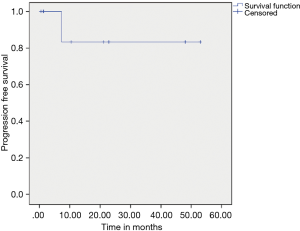
Patients who received adjuvant IM did better than those who did not on univariate and multivariate analysis respectively (P=0.025 and P=0.2) (Figure 5). On multivariate analysis only adjuvant imatinib was found to significant for DFS with P value 0.041. The estimated 5-year PFS and OS was found to be 88.8% and 100% respectively.
Discussion
GISTs are the most common mesenchymal tumors of GI tract. The incidence may be underestimated in Indian population. Though a rare tumor, high rates of cure and improved OS is currently the norm with IM and multimodality therapy.
The median age at diagnosis is usually 60 years while in our population median age is around 54 years which has been reported in other series as well (16,17). Our study shows male to female preponderance with a ratio of 2.4: while most population based studies shows no sex predilection (17-19).
Stomach is the most common site with 60%, while small bowel constitutes 20–30% in western countries (20,21). Studies from Japan also supports stomach as the most common site (up to 70%) (22). Our study showed a slightly lesser proportion of gastric GIST and an increased number of small bowel GIST, but this could be a referral bias.
Very low & low risk constitute a very small percentage in our study, while most patients belong to high risk category in our group (again can be a referral bias) as compared to other studies published in literature (23,24). In our study there was no statistical significant difference in DFS & OS as per risk stratification. This is likely because most of the patients present to our institute with larger tumor size and advanced presentation which adds to selection bias in risk stratification distribution. The addition of neoadjuvant IM in a majority of our patients may dilute the effect of risk stratification.
The optimal duration of neoadjuvant IM therapy is not clearly defined and usually ranges from 4–12 months (11,25). Within this duration, optimal response is usually achieved, risk of developing secondary resistance is low and best results can be achieved with surgery. The German phase II CST1571-BDE43 trial of preoperative IM (given for 6 months) shows that preoperative IM therapy improves the resectability rate and organ preservation, with 64% patients undergoing less extensive resection post neoadjuvant IM than initially planned (15). In a pooled database of ten EORTC STBSG sarcoma centers, 161 patients with locally advanced GIST who received neo-adjuvant IM were studied. After a median 40 weeks of IM, the rate of R0 resection was 83% and the 5-year DFS was 65% with median OS of 104 months (13). Even with a majority of the tumor cohort in our study being bulky at baseline, within a median of 5 months neoadjuvant IM, they become operable. Median duration of neoadjuvant imatinib therapy was 5 months (1–98 months) in our data which is consistent with Phase III EORTC trial (14). Patients on neoadjuvant IM yet to undergo surgical evaluation are under periodic monitoring with a median follow up of 6 months. However, it may be prudent to consider longer duration of neoadjuvant imatinib before declaring unresectability or achieving adequate resectability. This is supported by the fact that the German study showed 64% resectability with median neoadjuvant imatinib of 6-month versus EORTC study showed 83% resectability with median neoadjuvant imatinib of 10 months (13,15).
CR was achieved only in 1 patient while most of the patients (64.50%) had PR to preoperative imatinib therapy. A total of 29% of patients had SD while 4 patients progressed locally while on neoadjuvant therapy. Our results differ from the RTOG 0132 phase 2 trial in which 83% of patients had SD amongst the 31 patients with primary GIST and lesser PR rates. This is possibly due to the shorter duration of neoadjuvant IM given in this trial (8–12 weeks) compared to our study (12).
The prognostic factor that was consistently associated with PFS and OS in our data was the administration of adjuvant IM. As the value of risk stratification post neoadjuvant IM is still unclear and a majority of our patients were locally advanced having received IM neoadjuvant, further studies are required to evaluate prognostic factors in patients who receive neoadjuvant therapy. So, in spite of neoadjuvant IM, adjuvant imatinib has remained a prognostic variable in multivariate analysis. Decision of adjuvant imatinib based on post op risk stratification may not be applicable after neoadjuvant IM.
The ACOSOG Z9001 trial shows improved PFS of 98% vs. 83% in patients with tumor size >3 cm and who received adjuvant IM therapy for 1 year as compared to placebo arm; however there was no difference in OS (5). In our series 82.50% of patients received adjuvant imatinib therapy with median duration of 21 months with estimated 3- & 5-year DFS & OS 86.10% & 67% and 95.40% & 91.70% respectively. The results are consistent with the Scandinavian-German SSGXVIII/AIO trial where patients who received 36 months of adjuvant imatinib therapy had better RFS 65.60% vs. 47.90% (HR 0.46) and OS 92% vs. 81.7% (HR 0.45) respectively than patients who received 12 months of adjuvant therapy (7).
The responses seen in our metastatic patients provide an interesting option in a potentially select group who do not have extensive metastases and respond well to IM therapy. Even in presence of peritoneal metastasis, considering surgery for primary along with oligo metastasis after anterior IM therapy should be considered. However, these are small numbers and the benefit of surgery in patients with metastatic GISTs still remains controversial (26,27).
Within the confines of a retrospective analysis, our data throws light over the distribution of patients with GIST in India and their responses to therapy. The major takeaway remains the excellent responses seen with neoadjuvant IM, low rate of progression on IM, need of adjuvant IM post neoadjuvant use and long term outcomes comparable with international data.
The major drawback of this analysis is its focus on operated patients. It does not provide information on the number of patients who actually respond to neoadjuvant IM as a proportion of patients receiving neoadjuvant IM as a whole as this data is yet to mature.
Conclusions
Standardization of clinical, surgical, radiological & pathological assessment with multidisciplinary approach improves the outcomes in management of GISTs. Neoadjuvant IM therapy improves resectability rate with good responses, even in patients with bulky disease as evinced by our data. Newer prognostic variables require validation in patients undergoing neoadjuvant IM. Adjuvant IM therapy should be considered in all intermediate & high risk patients post-surgery and those who received neoadjuvant IM. Identification of patients with significant response to IM therapy and appropriate selection of patients with metastatic disease at presentation for surgical resection may improve outcome in this subgroup of patients.
Acknowledgements
None.
Footnote
Conflict of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by institutional ethics committee/ethics board (No. IEC/0815/1524/001).
References
- Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med 2006;130:1466-78. [PubMed]
- Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol 2002;33:459-65. [Crossref] [PubMed]
- Roberts PJ, Eisenberg B. Clinical presentation of gastrointestinal stromal tumors and treatment of operable disease. Eur J Cancer 2002;38:S37-8. [Crossref] [PubMed]
- Rutkowski P, Debiec-Rychter M, Ruka W. Gastrointestinal Stromal Tumors: Key to Diagnosis and Choice of Therapy. Mol Diagn Ther 2008;12:131-43. [Crossref] [PubMed]
- Dematteo RP, Ballman KV, Antonescu CR, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet 2009;373:1097-104. [Crossref] [PubMed]
- Joensuu H, Vehtari A, Riihimäki J, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol 2012;13:265-74. [Crossref] [PubMed]
- Joensuu H, Eriksson M, Sundby Hall K, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA 2012;307:1265-72. [Crossref] [PubMed]
- Shrikhande SV, Marda SS, Suradkar K, et al. Gastrointestinal stromal tumors: case series of 29 patients defining the role of imatinib prior to surgery. World J Surg 2012;36:864-71. [Crossref] [PubMed]
- Koontz MZ, Visser BM, Kunz PL. Neoadjuvant imatinib for borderline resectable GIST. J Natl Compr Cancer Netw 2012;10:1477-82; quiz 1482.
- Tielen R, Verhoef C, van Coevorden F, et al. Surgical treatment of locally advanced, non-metastatic, gastrointestinal stromal tumours after treatment with imatinib. Eur J Surg Oncol 2013;39:150-5. [Crossref] [PubMed]
- McAuliffe JC, Hunt KK, Lazar AJ, et al. A randomized, phase II study of preoperative plus postoperative imatinib in GIST: evidence of rapid radiographic response and temporal induction of tumor cell apoptosis. Ann Surg Oncol 2009;16:910-9. [Crossref] [PubMed]
- Wang D, Zhang Q, Blanke CD, et al. Phase II trial of neoadjuvant/adjuvant imatinib mesylate for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumors: long-term follow-up results of Radiation Therapy Oncology Group 0132. Ann Surg Oncol 2012;19:1074-80. [Crossref] [PubMed]
- Rutkowski P, Gronchi A, Hohenberger P, et al. Neoadjuvant imatinib in locally advanced gastrointestinal stromal tumors (GIST): the EORTC STBSG experience. Ann Surg Oncol 2013;20:2937-43. [Crossref] [PubMed]
- Le Cesne A, Van Glabbeke M, Verweij J, et al. Absence of progression as assessed by response evaluation criteria in solid tumors predicts survival in advanced GI stromal tumors treated with imatinib mesylate: the intergroup EORTC-ISG-AGITG phase III trial. J Clin Oncol 2009;27:3969-74. [Crossref] [PubMed]
- Hohenberger P, Langer C, Wendtner CM, et al. Neoadjuvant treatment of locally advanced GIST: Results of APOLLON, a prospective, open label phase II study in KIT- or PDGFRA-positive tumors. J Clin Oncol 2012;30:abstr 10031.
- Bülbül Doğusoy DG. Gastrointestinal stromal tumors: A multicenter study of 1160 Turkish cases. Turk J Gastroenterol 2012;23:203-11. [Crossref] [PubMed]
- Lv M, Wu C, Zheng Y, et al. Incidence and Survival Analysis of Gastrointestinal Stromal Tumors in Shanghai: A Population-Based Study from 2001 to 2010, Incidence and Survival Analysis of Gastrointestinal Stromal Tumors in Shanghai: A Population-Based Study from 2001 to 2010. Gastroenterol Res Pract 2014;2014:e834136.
- Alvarado-Cabrero I, Vázquez G, Sierra Santiesteban FI, et al. Clinicopathologic study of 275 cases of gastrointestinal stromal tumors: the experience at 3 large medical centers in Mexico. Ann Diagn Pathol 2007;11:39-45. [Crossref] [PubMed]
- Mucciarini C, Rossi G, Bertolini F, et al. Incidence and clinicopathologic features of gastrointestinal stromal tumors. A population-based study. BMC Cancer 2007;7:230. [Crossref] [PubMed]
- Caterino S, Lorenzo L, Petrucciani N, et al. Gastrointestinal stromal tumors: correlation between symptoms at presentation, tumor location and prognostic factors in 47 consecutive patients. World J Surg Oncol 2011;9:13. [Crossref] [PubMed]
- Bhalgami R, Manish K, Patil P, et al. Clinicopathological study of 113 gastrointestinal stromal tumors. Indian J Gastroenterol 2013;32:22-7. [Crossref] [PubMed]
- Terada T. Gastrointestinal stromal tumor of the digestive organs: a histopathologic study of 31 cases in a single Japanese institute. Int J Clin Exp Pathol 2009;3:162-8. [PubMed]
- Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol 2008;39:1411-9. [Crossref] [PubMed]
- Brabec P, Sufliarsky J, Linke Z, et al. A whole population study of gastrointestinal stromal tumors in the Czech Republic and Slovakia. Neoplasma 2009;56:459-64. [Crossref] [PubMed]
- Haller F, Detken S, Schulten HJ, et al. Surgical management after neoadjuvant imatinib therapy in gastrointestinal stromal tumours (GISTs) with respect to imatinib resistance caused by secondary KIT mutations. Ann Surg Oncol 2007;14:526-32. [Crossref] [PubMed]
- Gronchi A, Fiore M, Miselli F, et al. Surgery of residual disease following molecular-targeted therapy with imatinib mesylate in advanced/metastatic GIST. Ann Surg 2007;245:341-6. [Crossref] [PubMed]
- Park SJ, Ryu MH, Ryoo BY, et al. The role of surgical resection following imatinib treatment in patients with recurrent or metastatic gastrointestinal stromal tumors: results of propensity score analyses. Ann Surg Oncol 2014;21:4211-7. [Crossref] [PubMed]


