Post operative stereotactic radiosurgery for positive or close margins after preoperative chemoradiation and surgery for rectal cancer
Introduction
Advances in surgical technique (1) and preoperative therapy (2,3) have made a significant impact in reducing postoperative positive margins and local recurrences in rectal cancer. Patients may still have positive resection margins resulting in an overall local recurrence rate between 5–15% (1,3,4) and a poorer overall survival rate (5) making resection margin status an important prognostic factor. After neoadjuvant radiation, it is unclear what the best local therapy approach for positive margin is after surgery for rectal cancer. Further surgery at recurrence may often not be feasible and have excessive morbidity (6,7). Additional external beam radiation therapy (EBRT) can be unsafe as dose limiting normal tissues in pelvis such as the bowel or the bladder have often been treated to tolerance (8). Intraoperative radiation therapy (IORT) (9) and brachytherapy (10) has been used when positive margins are anticipated at the time of surgery, but often microscopic positive margins are unsuspected.
Stereotactic Body Radiotherapy (SBRT) has been successfully used in the primary therapy or as a boost due to its conformality and sharp dose fall off thereby avoiding dose limiting normal structures of normal structures (11,12). Hence it is conceivable that SBRT may be applicable for positive margins in patients with rectal cancer who have had neoadjuvant radiation. We report our experience in the use of SBRT as a boost to positive margins after prior radiation.
Methods
Patients and lesions
Our institutional IRB approved database (DFHCC 09-451) was retrospectively reviewed. Rectal carcinoma patients previously treated with radiation, with positive margins after surgery, who were treated with SBRT, were included in this study. Seven patients were identified between February 2006 and December 2012.
SBRT treatment
At least three gold fiducial markers were placed at the area of concern during surgery. All patients underwent CT simulation in supine position with VacLocTM body immobilization system. Intravenous and rectal barium contrast agent was used during CT planning and 1 mm axial CT images were obtained in the region of interest. The gross tumor volume (GTV) and was contoured on axial CT images. The MultiplanTM software was used for treatment planning and all patients were treated with the CyberknifeTM system with real time fiducial tracking. The pathology report was correlated with the fiducial markets to identify the target volume. The dose fractionation scheme was individualized based mostly on tumor volume and location of the tumor. Other factors, such as, patients’ performance and comorbidity were also taken into consideration.
Close/positive margins
For this study positive margin was described as being ≤1 mm and close margins was defined as cancer cells within 1–2 mm.
Follow-up
Follow-up data included status of disease, date of progression if any, site of failure and last follow up date. Routine follow-up included CT imaging at 1 month after treatment and 3–6 months thereafter. Local failure was defined as clinical or radiological evidence of pelvic recurrence. If a new lesion developed in the pelvis but outside the radiation field, it was interpreted as failure outside the treated area. Acute and late toxicities were defined as symptoms that develop within three months after SBRT or later, respectively.
Statistics
Descriptive statistics was used to describe the data. Kaplan-Meir actuarial curves were used to estimate local control and overall survival. GraphPad Prism Version 6.0.c software (GraphPad Software, Inc., La Jolla, CA, USA) was used for statistical analysis.
Results
Pretreatment characteristics
Seven patients were treated with SBRT boost with the CyberknifeTM system for positive margins at our center between February 2006 and December 2012. Three males and four females were included. The median age at the time of reirradiation was treatment was 65 years (range, 48–79 years).
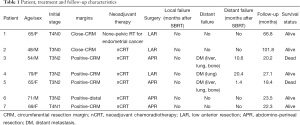
Initial stages at presentation were as follows: T4N1 in one patient, T4N0 in one patient, T3N2 in four patients and T3N0 in two patients. As initial surgery, four patients underwent abdomino-perineal resection (APR), three had low anterior resection (LAR). All patients had previously received EBRT, six as part of neoadjuvant therapy and one patient had pelvic irradiation for endometrial cancer before she was diagnosed with rectal cancer. For the eight lesions treated the prior radiation dose received was 50.4 Gy. The patient characteristics are described in Table 1.
Full table
SBRT treatment
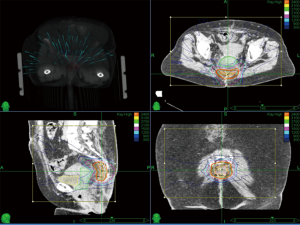
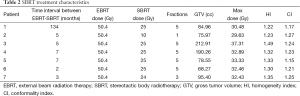
The median tumor volume was 84.96 cc (range, 68.27-212.91 cc). The median prescription isodose was 78% (range, 69–86%). Total SBRT dose ranged from 10 to 25 Gy (median 25 Gy) in fractions ranging from 1 to 5 (median 5). Median conformality index (CI) and homogeneity index (HI) was 1.23 and 1.32 respectively. The treatment characteristics are described in Table 2. A representative treatment plan is shown in Figure 1.
Full table
Efficacy
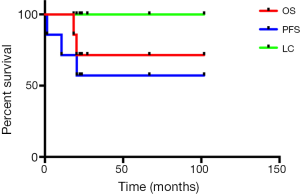
Median follow-up from the time of SBRT was 23.5 months (18.4 to 101.8 months). The actuarial 1- and 2-year survival rates were 100%, 71% respectively. The long-term local control rates were 100%. Local progression free survival rates at 1 and 2 year were 71% and 57% respectively. The follow-up data is also summarized in Table 1. Actuarial local control, progression free survival and overall survival are shown in Figure 2.
Toxicity
Most patients (6 out of 7) developed fatigue. Two patients had diarrhea, which was controlled with medication not requiring further treatment.
Discussion
Fortunately, neoadjuvant radiation and chemoradiation and improved surgical techniques have significantly decreased the likelihood of positive margins. However, when they do occur in 5-15% of patients, it can be a therapeutic challenge. We have shown in this series that planned SBRT can be a safe and useful technique to salvage positive margins after neoadjuvant chemoradiation and surgery. In our case series of seven patients who had SBRT treatments for positive margins, we observed a 100% local control for long term and overall survival of 100% in 1 year and 71% in 2 year, with only Grade II toxicity.
It is well known that positive margins after surgery is a poor prognostic factor for local recurrence and survival (5,13). With upfront surgery, postoperative chemoradiation is often used to in patients with positive margins (14) to improve local control and survival. However in the setting of neoadjuvant chemoradiation, the significance of a positive margin is unclear and the management can be challenging. When patients are at a high risk for positive margins, in the locally advanced or recurrent setting, traditionally IORT (15) and brachytherapy (10) has been used. Even if these techniques are available, often-microscopic margins are not anticipated until full pathological review. Notwithstanding, apart from technical challenges, IORT and brachytherapy are not without significant toxicity (16,17). Conventional external beam radiation in this setting is often limited by tolerance to previously radiated pelvic tissues, particularly bowel.
Salvage after pelvic failure can be formidable, particularly after prior radiation therapy. More often after initial combined modality therapy, pelvic failures are salvaged by further surgery (18,19) with or without further radiation (20). However this is often not feasible without exenteration (21) and is associated with significant morbidity (16,17). IORT, brachytherapy, described above, and carbon ion therapy (22) have been used, but they are limited by availability and toxicity. Hence it makes sound clinical sense to prevent pelvic recurrences in the setting of positive margins after neoadjuvant chemoradiation and surgery.
SBRT has been used in the setting of pelvic recurrences after prior radiation (23-25). While these studies have shown that SBRT can be done safely, it is not curative in the treatment of gross recurrence. Hence we utilized SBRT electively to prevent pelvic failures in patients who had positive margins after neoadjuvant chemoradiation and surgery. This is a small retrospective series showing safety and efficacy of SBRT boost for positive margins after prior pelvic radiation, and further prospective studies need to be done to evaluate this approach.
Conclusions
After neoadjuvant chemoradiation and surgery close or positive microscopic margins can potentially lead to pelvic failure. Salvage may not be curative and associated with toxicity. In this first report of its kind, we have shown SBRT in this setting can safely decrease the likelihood of pelvic failure.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Havenga K, Enker WE, Norstein J, et al. Improved survival and local control after total mesorectal excision or D3 lymphadenectomy in the treatment of primary rectal cancer: an international analysis of 1411 patients. Eur J Surg Oncol 1999;25:368-74. [Crossref] [PubMed]
- Folkesson J, Birgisson H, Pahlman L, et al. Swedish Rectal Cancer Trial: long lasting benefits from radiotherapy on survival and local recurrence rate. J Clin Oncol 2005;23:5644-50. [Crossref] [PubMed]
- Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731-40. [Crossref] [PubMed]
- Kapiteijn E, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 2001;345:638-46. [Crossref] [PubMed]
- Baik SH, Kim NK, Lee YC, et al. Prognostic significance of circumferential resection margin following total mesorectal excision and adjuvant chemoradiotherapy in patients with rectal cancer. Ann Surg Oncol 2007;14:462-9. [Crossref] [PubMed]
- Akasu T, Yamaguchi T, Fujimoto Y, et al. Abdominal sacral resection for posterior pelvic recurrence of rectal carcinoma: analyses of prognostic factors and recurrence patterns. Ann Surg Oncol 2007;14:74-83. [Crossref] [PubMed]
- Huh JW. Curative potential of surgical resection for locally recurrent rectal cancer. Ann Surg 2014;259:e88. [Crossref] [PubMed]
- Yu SK, Bhangu A, Tait DM, et al. Chemoradiotherapy response in recurrent rectal cancer. Cancer Med 2014;3:111-7. [Crossref] [PubMed]
- Guo S, Reddy CA, Kolar M, et al. Intraoperative radiation therapy with the photon radiosurgery system in locally advanced and recurrent rectal cancer: retrospective review of the Cleveland clinic experience. Radiat Oncol 2012;7:110. [Crossref] [PubMed]
- Turley RS, Czito BG, Haney JC, et al. Intraoperative pelvic brachytherapy for treatment of locally advanced or recurrent colorectal cancer. Tech Coloproctol 2013;17:95-100. [Crossref] [PubMed]
- Mahadevan A, Floyd S, Wong E, et al. Stereotactic body radiotherapy reirradiation for recurrent epidural spinal metastases. Int J Radiat Oncol Biol Phys 2011;81:1500-5. [Crossref] [PubMed]
- Mahadevan A, Miksad R, Goldstein M, et al. Induction gemcitabine and stereotactic body radiotherapy for locally advanced nonmetastatic pancreas cancer. Int J Radiat Oncol Biol Phys 2011;81:e615-22. [Crossref] [PubMed]
- Arumugam PJ, Vivek V, Beynon J. Prognostic significance of the circumferential resection margin following total mesorectal excision for rectal cancer (Br J Surg 2002; 89: 327-34). Br J Surg 2002;89:1067; author reply 1067. [Crossref] [PubMed]
- Fisher B, Wolmark N, Rockette H, et al. Postoperative adjuvant chemotherapy or radiation therapy for rectal cancer: results from NSABP protocol R-01. J Natl Cancer Inst 1988;80:21-9. [Crossref] [PubMed]
- Pezner RD, Chu DZ, Ellenhorn JD. Intraoperative radiation therapy for patients with recurrent rectal and sigmoid colon cancer in previously irradiated fields. Radiother Oncol 2002;64:47-52. [Crossref] [PubMed]
- Klink CD, Binnebösel M, Holy R, et al. Influence of intraoperative radiotherapy (IORT) on perioperative outcome after surgical resection of rectal cancer. World J Surg 2014;38:992-6. [Crossref] [PubMed]
- Mannaerts GH, Schijven MP, Hendrikx A, et al. Urologic and sexual morbidity following multimodality treatment for locally advanced primary and locally recurrent rectal cancer. Eur J Surg Oncol 2001;27:265-72. [Crossref] [PubMed]
- Alberda WJ, Verhoef C, Nuyttens JJ, et al. Outcome in patients with resectable locally recurrent rectal cancer after total mesorectal excision with and without previous neoadjuvant radiotherapy for the primary rectal tumor. Ann Surg Oncol 2014;21:520-6. [Crossref] [PubMed]
- Mohan HM, Evans MD, Larkin JO, et al. Multivisceral resection in colorectal cancer: a systematic review. Ann Surg Oncol 2013;20:2929-36. [Crossref] [PubMed]
- Valentini V, Morganti AG, Gambacorta MA, et al. Preoperative hyperfractionated chemoradiation for locally recurrent rectal cancer in patients previously irradiated to the pelvis: A multicentric phase II study. Int J Radiat Oncol Biol Phys 2006;64:1129-39. [Crossref] [PubMed]
- Ferenschild FT, Vermaas M, Verhoef C, et al. Total pelvic exenteration for primary and recurrent malignancies. World J Surg 2009;33:1502-8. [Crossref] [PubMed]
- Combs SE, Kieser M, Habermehl D, et al. Phase I/II trial evaluating carbon ion radiotherapy for the treatment of recurrent rectal cancer: the PANDORA-01 trial. BMC Cancer 2012;12:137. [Crossref] [PubMed]
- Dagoglu N, Mahadevan A, Nedea E, et al. Stereotactic body radiotherapy (SBRT) reirradiation for pelvic recurrence from colorectal cancer. J Surg Oncol 2015;111:478-82. [Crossref] [PubMed]
- Defoe SG, Bernard ME, Rwigema JC, et al. Stereotactic body radiotherapy for the treatment of presacral recurrences from rectal cancers. J Cancer Res Ther 2011;7:408-11. [Crossref] [PubMed]
- Abusaris H, Hoogeman M, Nuyttens JJ. Re-irradiation: outcome, cumulative dose and toxicity in patients retreated with stereotactic radiotherapy in the abdominal or pelvic region. Technol Cancer Res Treat 2012;11:591-7. [PubMed]