Neoadjuvant vs. adjuvant treatment of Siewert type II gastroesophageal junction cancer: an analysis of data from the surveillance, epidemiology, and end results (SEER) registry
Introduction
Gastroesophageal junction (GEJ) cancer encompasses the distal 5 cm of the esophagus to the proximal 5 cm of the stomach, and are further subdivided based on the Siewert classification which was introduced in 1987. Siewert I cancer arises from the distal esophagus, Siewert II cancer arises from the true gastric cardia, and Siewert III cancer is subcardinal (1-3). Given that the incidence of GEJ cancer has risen by an estimated 350% since the 1970’s, determination of optimal treatment is imperative to improve patient outcomes (4-7).
With surgery alone, GEJ cancer has a 5-year survival rate of less than 30%, mostly due to the high risk of recurrence and distant metastasis (8,9). In general, the surgical and adjuvant treatment for Siewert I mirrors that of esophageal cancer, and the surgical and adjuvant treatment for Siewert III mirrors that of gastric cancer (10-12). Although the surgical management for Siewert II cancer is similar to Siewert III due to similarities in lymphatic spread (2,10,13), the optimal adjuvant therapy for Siewert II cancer is yet to be determined. This is partly due to the fact that the largest trials exploring optimal treatment for esophageal cancer and gastric cancer both include cancer of the GEJ (5,14).
The main trial advocating for adjuvant chemoradiotherapy (CRT) of GEJ and gastric adenocarcinoma was Intergroup trial 0116 (INT-0116), which concluded that there is a strong overall and relapse-free survival advantage for gastric and GEJ adenocarcinoma patients treated with adjuvant CRT over surgery alone (12). The findings of the trial have set the standard of care since 2001, and many groups have adopted it for gastric cancer although the standard of care for GEJ cancer has remained controversial. Interestingly, in 2010 the CROSS trial revealed that neoadjuvant CRT was superior to surgery alone for patients with adenocarcinoma of the distal esophagus or GEJ (11).
To date, prospective trials have demonstrated that there is value in the use of adjuvant and neoadjuvant CRT in the treatment of GEJ cancer. However, it remains unclear which approach is superior specifically for cancer of the true gastric cardia (Siewert type II). Here we study a large population of patients solely with Siewert II GEJ cancer. Using data from the surveillance, epidemiology, and end results (SEER) registry of the National Cancer Institute from 2001 to 2011, we compared survival in Siewert class II GEJ cancer patients treated with neoadjuvant versus adjuvant therapy. Since the SEER database collects information on radiation but not chemotherapy, we used the administration of neoadjuvant or adjuvant RT as proxy for combined modality treatment. Given that the standard of care has been concurrent CRT, we felt is safe to assume that patients treated after 2001 received combined modality treatment. The purpose of this study is to determine whether adjuvant or neoadjuvant therapy is associated with better outcomes specifically for Siewert type II cancer of the GEJ.
Methods
The SEER registry compiles cancer incidence, treatment, and survival data from 18 population-based cancer registries, covering more than 25% of the population of the United States. The SEER registry was accessed to identify all patients diagnosed with cancer of the GEJ from 2001 (year of publication of Intergroup trial 0116 results) until 2011 (most recent available data in the database). The variables obtained for each case include patient demographics (race/ethnicity, sex, age at presentation, year of diagnosis), disease characteristics (histologic grade, surgical stage/extent of disease, nodal status of the disease, presence of distant metastases), and treatment modalities (radiation sequence relative to surgery, type of surgery performed, and type of radiation administered). Cancer is a reportable disease under the laws of all 50 U.S. states and thus informed consent by all subjects is not required. All of the SEER data are de-identified and analysis of the data does not require IRB approval.
Although the SEER database did not specifically report the Siewert classification of GEJ cancer (i.e., type I, type II, or type III), we were able to translate the definition of a Siewert type II cancer into the variables encoded in the SEER database. There are two major variables in the SEER database that code for the tumor location (“CS Schema v0204” and “Primary Site - Labeled”) that differ in their descriptive terminology. In order to be as specific as possible, patients included in our cohort had to satisfy two conditions: both a CS Schema entry of “EsophagusGEJunction” and a Primary Site entry of “Cardia, NOS.” Of note, other entry choices for CS Schema include “Esophagus” and “Stomach,” and other entry choices for Primary Site include “Lower Third of the Esophagus,” “Esophagus NOS,” and other parts of the stomach such “Fundus of Stomach” and “Body of Stomach.” The project was discussed with SEER personnel who confirmed that this query was specific to Siewert type II cancer.
Patient population
The total number of cases with both a primary site described as “Esophagus GE Junction” and a collaborative stage schema described as “Cardia, NOS” (i.e., Siewert type II) obtained from SEER was 31,935. Patients with multiple primary malignancies (5,558), metastatic disease (3,759), unknown stage group (1,727), stage group IA (2,012), unknown grade (5,568), no cancer-directed surgery (9,910), no radiation therapy (RT) (16,505), or cases where it was unknown if RT was administered (1,012) were excluded from the study. Stage group IA was removed since this group was not included in the Intergroup 0116 trial, and these patients were more likely to receive surgery alone. We also removed all surgeries described as biopsy, nodal surgery only, photodynamic therapy, cryotherapy, electrocautery, surgery NOS, and polypectomy, leading to a final cohort of 1,497 patients. The extent of disease of patients diagnosed prior to 2004 was not based on a TNM staging system, but rather on a descriptive classification (ex. “regional nodes involved”). Because of this, many cases prior to 2004 were excluded due to incomplete data on the exact number of lymph nodes involved, and thus an inability to categorize the case into an AJCC 7th edition stage grouping for gastric cancer. The staging for gastric cancer was chosen as this was the convention used by the SEER database when staging GE junction cancer. Patients involved in the cohort had a variety of different surgeries, but mostly subtotal or total gastrectomy with or without removal of a portion of the distal esophagus.
Statistical analysis
Chi-square test with exact P values based on Monte Carlo simulation and Student’s t-test were used to compare categorical variables and continuous variables, respectively. Overall survival (OS) is defined as the time (months) from the surgery to the last follow-up or death whichever occurs first. The disease-specific survival (DSS) is defined as the time (months) from diagnosis to last follow-up or death, whichever occurs first, with death because of other reason treated as censored. OS and DSS were compared between patients with neoadjuvant and adjuvant radiation using log-rank test. Reported median follow-up time is based on the Kaplan-Meier estimate of potential follow-up (15). The comparison were further studied using multiple Cox proportional hazard regression models to adjust for age at diagnosis, year of diagnosis (2001–2009 vs. 2010–2011), sex, race, grade, histology, and stage. The proportional hazard assumption for Cox model was confirmed. Estimated hazard ratios (HRs) for each category of the variable versus the reference level and their 95% confidence intervals were reported. Statistical analysis was performed using SAS 9.3 (SAS Institute, Inc., Cary, NC, USA) and statistical significance was set at 0.05.
Results
Table 1 shows the descriptive table for categorical and continuous variables classified by radiation sequence with surgery, respectively. The table suggested that sex, race, grade, year of diagnosis, stage, vital status, and cause specific death were significantly associated with radiation sequence.
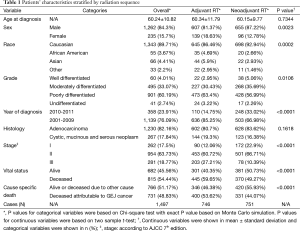
Full table
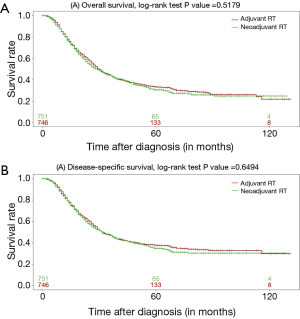
Figure 1A and1B show the Kaplan-Meier curves for OS and DSS, respectively. Log-rank test suggested the radiation sequence was not marginally associated with survival (P value =0.5179 and 0.6494 for OS and DSS comparison, respectively). The median OS time for patients with neoadjuvant RT was 29 months (95% CI: 25, 33) while the median OS time for patients with adjuvant RT was 30 months (95% CI: 28, 34). The median follow-up times for the neoadjuvant and adjuvant radiation cohorts were 41 and 63 months, respectively.
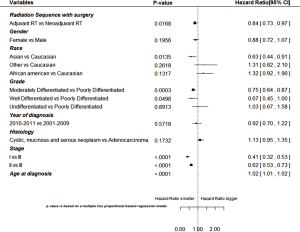
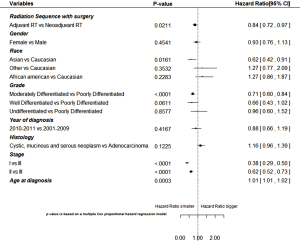
Figures 2 and3 show the estimated HRs and corresponding 95% confidence intervals for variables based on multivariable cox proportional HR model for OS and DSS, respectively. Patients with adjuvant radiation had significantly lower death risk and disease-specific risk than patients with neoadjuvant radiation after controlling for other covariates. Race (Asian vs. White), grade (well differentiated vs. poorly differentiated and moderately differentiated vs. poorly differentiated), stage group (I vs. III and II vs. III), and younger age of diagnosis were also significantly associated with both increased OS and DSS after controlling for all other variables in the multivariable analysis.
Discussion
The use of neoadjuvant or adjuvant CRT in the treatment of GEJ cancer has been previously studied, but the largest studies have combined GEJ cancer with either gastric or esophageal cancer. In 2001, Intergroup trial 0116 enrolled a total of 556 patients, approximately 20% of them had adenocarcinoma of the GEJ while the remainder had gastric adenocarcinoma. Although the study does not mention the Siewert classification, it is likely that they excluded Siewert I since these are distal esophageal cancers. The study showed survival benefit for patients receiving adjuvant CRT compared with surgery alone (12,16).
The CROSS trial is the largest study to date (n=366) investigating the use of neoadjuvant CRT for the treatment of cancers of the distal esophagus or GEJ. Approximately 24% of the patients enrolled in this study had cancer of the GEJ. This study does not use Siewert classification, although it is likely that they included only Siewert I and II since their description of the GEJ cancer was “tumors involving both the esophagus and the cardia on histology.” The study showed increased OS in patients treated with neoadjuvant CRT compared to surgery alone, but this was strongly driven by the 23% of patients with squamous cell carcinoma. When isolating the population of patients with adenocarcinoma, the benefits only approached significance (P=0.07) (11,16).
The only phase III trials exploring CRT in patients solely with GEJ cancer is reported by Stahl et al. The study accrued 119 patients with locally advanced adenocarcinoma of the GEJ (Siewert types I, II, and III) and compared induction chemotherapy plus surgery to neoadjuvant CRT plus surgery. Although the study was closed early, the neoadjuvant CRT group showed an improved 3-year survival that approached significance (P=0.07). The authors do not analyze survival after stratifying patients by Siewert class (17). There are also several ongoing trials currently evaluating CRT for patients with esophageal and GEJ adenocarcinoma and/or squamous cell carcinoma. The QUINTETT trial evaluating the use of surgery plus neoadjuvant CRT compared to surgery plus adjuvant CRT (18). Also, a trial from Zhejiang Cancer Hospital is evaluating surgery plus neoadjuvant CRT with consolidative chemotherapy compared to surgery plus adjuvant CRT with consolidative chemotherapy (19).
In general, Siewert type I cancer is epidemiologically and histologically similar to esophageal cancer, and Siewert type III cancer is epidemiologically and histologically similar to gastric cancer. As such, the adjuvant treatments for these cancers typically mirror the treatment for esophageal and gastric cancer, respectively. Since Siewert type II cancers are in the transition zone between the esophagus and stomach (i.e., true gastric cardia), the optimal adjuvant treatment is unknown. In the present study, we demonstrate for the first time a benefit to adjuvant RT in the treatment of Siewert type II cancer of the GEJ. The data show a lower death risk and lower disease-specific death risk for adjuvant RT compared to neoadjuvant RT after controlling for other risk factors in the multivariable Cox proportional hazard regression model. Although Table 1 shows the adjuvant RT cohort to have more deaths attributable to GEJ cancer, this is likely due to a combination of a longer median follow up time and a higher portion of stage III disease in the adjuvant cohort.
It is interesting that we found adjuvant RT to be associated with a survival benefit given the theoretical benefits of neoadjuvant treatment. Firstly, the treatment is likely to be better tolerated since patients are not facing the morbidity associated with a recent major surgery. This conjecture is supported by the fact that over 90% of patients in the CROSS trial completed neoadjuvant CRT, whereas only 64% of patients completed adjuvant CRT in the INT-0116 trial. In addition, there is a possibility that radiation treatment may be less effective in the adjuvant setting due to the alterations in blood flow associated with surgical resection. Potentially, alterations in blood flow to the surgical site can lead to decreased oxygenation of the tumor bed, and thus a lesser effect of radiation treatment. Another potential advantage is that there is a clearer radiation target in the neoadjuvant setting as compared to the adjuvant setting—this can lead to smaller field sizes, and possibly less toxicity associated with treatment. Finally, in the neoadjuvant setting, the irradiated tissues are removed and thus there is a lesser chance of secondary malignancy should the patient achieve long term survival.
A possible rationale for the favorable association between survival and adjuvant RT that we described herein may lay within the degree of nodal coverage in the radiation fields associated with the CROSS and INT-0116 trials. In 2008, Meier et al. described the risk of microscopic lymph node involvement of various nodal groups for 326 patients with GEJ cancer that had primary surgical resection and lymphadenectomy. Of note, patients with T2–T4 Siewert type II GEJ cancer had at least a 15–20% risk of microscopic nodal involvement in many nodal groups, including lower esophageal, paracardial, lesser curvature, greater curvature, left gastroepiploic, celiac trunk, left gastric, splenic artery, splenic hilar, and left paraaortic nodes (20). Interestingly, the field designs described in the INT-0116 trial protocol cover all of these nodal regions, whereas there was no treatment to nodal areas at risk in the CROSS protocol. Since these two major trials set the paradigms for both adjuvant and neoadjuvant treatment of the GEJ, there is a possibility that patients in the adjuvant treatment cohort of this study received radiation to these nodal groups, whereas the neoadjuvant treatment cohort did not. Since the SEER database does not provide information on radiation field design, this speculation cannot be proven at this juncture.
There are several limitations to this study. First, the SEER database provides no information on comorbidities or patient performance status, which may have influenced the type of treatment that these patients received. Another source of limitation in this study is the lack of information on the administration of chemotherapeutic agents in the SEER database. Since several trials and a meta-analysis have failed to prove that neoadjuvant radiation alone confers a survival benefit in esophageal cancer (21-23), we assume that the patients in the neoadjuvant RT cohort of the present study have received some form of chemotherapy. Also supporting this notion are the results of the CROSS and CALGB 9781 trials which advocate for neoadjuvant CRT in the treatment of esophageal cancer (11,24). In addition, it is reasonable to assume that patients in the adjuvant RT group also received chemotherapy due to the results of Intergroup trial 0116, as the regimen from this trial has been adopted as the standard of care throughout the country. SEER also lacks data on surgical technique and margin status as well as radiation dose, fields, length of treatment, and type of image guidance utilized. Thus, it is possible that the comparison of the cohorts in this study is not well balanced with respect to these unknown variables.
A potential source of bias in this study is the fact that the patients treated with neoadjuvant RT may have initially presented with a higher stage but were downstaged with neoadjuvant therapy. However, the number of patients receiving neoadjuvant therapy has increased over time (Table 1). This trend appears to parallel the publication of neoadjuvant trials. Thus one can speculate that clinicians have increasingly adopted neoadjuvant therapy as a means to improve outcomes, rather than simply for downstaging. Furthermore, the noted discrepancy between the univariate and multivariate analysis suggests that the adjuvant group, rather than the neoadjuvant group, may have actually had a higher proportion of patients with more advanced disease. The summary statistics (Table 1) seem to support this conjecture. If this were indeed true, the argument for adjuvant over neoadjuvant CRT becomes even stronger.
Conclusions
This is the first study based on retrospective review of a large population-based data registry to show an advantage to adjuvant over neoadjuvant therapy in the treatment of Siewert type II GEJ cancer. There are several potentially important variables that could not be considered in a SEER-based study, such as patient performance status, comorbidities, chemotherapy regimen, as well as specifics of radiation treatment such as dose, fields, length of treatment, and type of image guidance utilized. Given the described limitations and retrospective nature of this study, these results are intended to be thought-provoking and suggest the need future prospective trials to determine the optimal adjuvant therapy for Siewert type II GEJ cancer. More importantly, it calls for a much-needed consensus on the best therapeutic approach to challenging cases of GEJ cancer given its rising incidence in the U.S.
Acknowledgements
The authors would like to acknowledge Stony Brook University Hospital for funding this study.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Blom RL, Bogush T, Brücher BL, et al. Therapeutic approaches to gastroesophageal junction adenocarcinomas. Ann N Y Acad Sci 2014;1325:197-210. [Crossref] [PubMed]
- Rüdiger Siewert J, Feith M, Werner M, et al. Adenocarcinoma of the esophagogastric junction: results of surgical therapy based on anatomical/topographic classification in 1,002 consecutive patients. Ann Surg 2000;232:353-61. [Crossref] [PubMed]
- Siewert JR, Hölscher AH, Becker K, et al. Cardia cancer: attempt at a therapeutically relevant classification. Chirurg 1987;58:25-32. [PubMed]
- Botterweck AA, Schouten LJ, Volovics A, et al. Trends in incidence of adenocarcinoma of the oesophagus and gastric cardia in ten European countries. Int J Epidemiol 2000;29:645-54. [Crossref] [PubMed]
- Carr RM, Lynch JP. At the crossroads in the management of gastroesophageal junction carcinomas-where do we go from here? Gastrointest Cancer Res 2008;2:253-5. [PubMed]
- Trivers KF, Sabatino SA, Stewart SL. Trends in esophageal cancer incidence by histology, United States, 1998-2003. Int J Cancer 2008;123:1422-8. [Crossref] [PubMed]
- Sehdev A, Catenacci DV. Gastroesophageal cancer: focus on epidemiology, classification, and staging. Discov Med 2013;16:103-11. [PubMed]
- Moehler M, Lyros O, Gockel I, et al. Multidisciplinary management of gastric and gastroesophageal cancers. World J Gastroenterol 2008;14:3773-80. [Crossref] [PubMed]
- Krapcho M. SEER Cancer Statistics Review, 1975-2003. 2006.
- von Rahden BH, Stein HJ, Siewert JR. Surgical management of esophagogastric junction tumors. World J Gastroenterol 2006;12:6608-13. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 2001;345:725-30. [Crossref] [PubMed]
- Siewert JR, Feith M, Stein HJ. Biologic and clinical variations of adenocarcinoma at the esophago-gastric junction: relevance of a topographic-anatomic subclassification. J Surg Oncol 2005;90:139-46; discussion 146. [Crossref] [PubMed]
- Sandler S. Esophagogastric junction and gastric adenocarcinoma: neoadjuvant and adjuvant therapy, and future directions. Oncology (Williston Park) 2014;28:505-12. [PubMed]
- Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials 1996;17:343-6. [Crossref] [PubMed]
- Moorcraft SY, Smyth EC, Cunningham D. Adjuvant or neoadjuvant therapy for operable esophagogastric cancer? Gastric Cancer 2015;18:1-10. [Crossref] [PubMed]
- Stahl M, Walz MK, Stuschke M, et al. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol 2009;27:851-6. [Crossref] [PubMed]
- Malthaner R. Prospective Randomized Phase III Trial Comparing Preoperative Chemoradiation Therapy (Cisplatin, 5-FU and Radiotherapy Followed by Surgery) to Surgery Followed by Postoperative Chemoradiation (Cisplatin, Epirubicin, 5-FU, Radiotherapy) for Esophageal Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT00907543
- Prospective Randomized Phase II Trial Comparing Preoperative Chemoradiotherapy (Paclitaxel and Carboplatin) Followed by Surgery to Surgery Followed by Postoperative Chemoradiotherapy (Paclitaxel and Carboplatin) for Esophageal Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT01463501
- Meier I, Merkel S, Papadopoulos T, et al. Adenocarcinoma of the esophagogastric junction: the pattern of metastatic lymph node dissemination as a rationale for elective lymphatic target volume definition. Int J Radiat Oncol Biol Phys 2008;70:1408-17. [Crossref] [PubMed]
- Vallböhmer D, Hölscher AH, DeMeester S, et al. A multicenter study of survival after neoadjuvant radiotherapy/emotherapy and esophagectomy for ypT0N0M0R0 esophageal cancer. Ann Surg 2010;252:744-9. [Crossref] [PubMed]
- Lee PC, Port JL, Paul S, et al. Predictors of long-term survival after resection of esophageal carcinoma with nonregional nodal metastases. Ann Thorac Surg 2009;88:186-92; discussion 192-3. [Crossref] [PubMed]
- Arnott SJ, Duncan W, Gignoux M, et al. Preoperative radiotherapy for esophageal carcinoma. Cochrane Database Syst Rev 2005.CD001799. [PubMed]
- Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol 2008;26:1086-92. [Crossref] [PubMed]


