
Is Endoscopic Ultrasound (EUS) necessary in the pre-therapeutic assessment of Barrett’s esophagus with early neoplasia?
Introduction
Barrett’s esophagus (BE) is a well-established risk factor for developing esophageal adenocarcinoma. Endoscopic surveillance programs have been established in order to detect the presence of neoplasia and lesions at potentially curative stages including high grade dysplasia (HGD) and intramucosal carcinoma (IMC) (1). The development of new endoscopic therapies, in particular endoscopic mucosal resection (EMR) and several ablative therapies, offers curative and minimally invasive treatment for HGD and IMC. Thus, accurate diagnosis of the depth of tumor invasion and presence of lymph node metastasis is essential in order to identify patients who are candidates for endoscopic treatments. Endoscopic ultrasound (EUS) is the most accurate tool for the TNM staging of esophageal neoplasms (2,3). Despite this, its utility in staging prior to endoscopic or surgical treatment in early Barrett’s neoplasia is still debatable. Some studies have demonstrated that EUS may overstage early lesions and is limited by operator experience, location and morphology of the lesions. Even with high frequency probes, it is difficult to distinguish HGD from IMC or cancer invading the submucosa (4-8). Our hypothesis is that EUS provides limited information in Barrett’s associated neoplasia and often overstages disease in tumor depth assessment. The aim of this study was to evaluate the utility of EUS in the pre-therapeutic phase of Barrett’s patients referred to a tertiary-care academic referral center being considered for treatment and the impact of information provided by EUS exams in making decisions for therapy.
Patients and methods
Data collection
All patients enrolled in a treatment protocol for Barrett’s esophagus neoplasia in our institution are included in an institutional review board approved prospective database. A systematic chart review was performed of all patients evaluated from January 1, 2001 to July 31, 2010. The patients eligible for inclusion were all those who had a EUS performed prior to treatment and a final histopathologic staging obtained by endoscopic mucosal resection or esophagectomy. After review, a total of 109 patients met the inclusion criteria and were included in the final analysis. Treatment indication, endoscopic findings, endosonographic findings, type of treatment and any subsequent pathology staging by endoscopic mucosal resection or surgical specimens were evaluated.
Endoscopy reports
Upper endoscopy was performed in every patient previous to or on the same day as the EUS, using either a standard or high-definition upper endoscope when available (GIF-Q160, GI-H180; Olympus America, Center Valley, PA) as well as narrow-band imaging (when available). All endoscopy reports were reviewed and the length of the Barrett’s segment, any visible lesions, and the location and estimated size of every lesion were noted. Visible lesions were categorized and recorded according to the Paris Classification for superficial neoplastic lesions (9). When EMR was performed, either curative intent or definitive histopathologic staging, a cap and snare technique, freehand, lift and cut or multi-band assisted method were used.
EUS reports
All endosonographic evaluations in cases with Barrett’s esophagus were carried out by two experienced interventional gastroenterologists who perform EUS on a routine basis. All exams were performed using a radial-scanning echo-endoscope (GF-UE160; Olympus America, Center Valley, PA).
The EUS reports were reviewed by two physicians who achieved consensus regarding the findings; in event of inconsistency, a third physician reviewed the case who served as the tie breaker. The endosonographic appearance of the esophageal wall (normal, diffuse thickening, focal thickening or invasive disease) and depth of the esophageal findings were recorded. Any peritumoral and celiac lymph nodes were considered suspicious for malignancy if two or more of the following criteria were met: size ≥10 mm, round shape, distinct borders, hypoechoic appearance, and heterogeneous aspect (3). Fine needle aspiration (FNA), if performed, and TNM staging by EUS were recorded.
EUS exams were categorized as having esophageal findings suspicious for invasion if they fulfilled one or more of the following criteria: EUS stage ≥T1bNxMx, thickening of the esophageal wall involving the submucosal layer, and presence of suspicious lymph nodes according to the endosonographic characteristics mentioned above. All EUS exams that did not fulfill at least one of the above criteria were considered as having negative esophageal findings.
Histopathologic staging
All pathology reports were reviewed by the same two physicians and the final staging according to the Vienna Classification of gastrointestinal epithelial neoplasia (10) was recorded. The results of cytological exam of FNA from lymph nodes when performed were also noted.
Statistical analyses
All continuous variables were summarized by their mean, median and range. Frequencies and percentages were reported for categorical variables.
Frequency distribution between two categorical variables was compared using a Chi square test for independence with Yate’s correction or a Fisher’s exact test.
Results
Characteristics of patients, procedures and pathology
Demographics and characteristics of the Barrett’s segment of all 109 eligible patients are summarized in Table 1. A male to female ratio of 4:1 was observed and long segment Barrett’s esophagus (LSBE), defined as being ≥3 cm, was seen in most of patients (median length 5 cm, mean 6.75 cm, range, 3-17 cm).
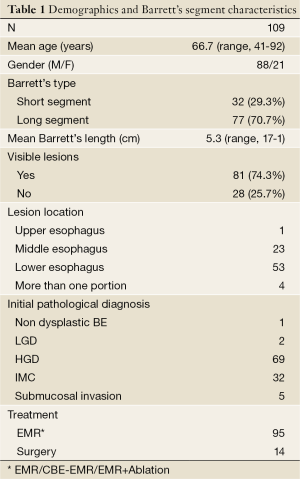
Full table
A total of 104 patients underwent EMR. Ninety-five patients underwent endoscopic resection with a curative intent: focal EMR =13, complete BE endoscopic mucosal resection (CBE-EMR) =56 and EMR of any visible lesion followed by ablation of the residual Barrett’s epithelium =26. Fourteen patients were referred to surgery for the following reasons: the diagnostic EMR samples had revealed at least submucosal invasion, risk factors for lymph node metastasis, or positive deep resection margins in 9 patients; EUS had suggested invasion in 4 patients, and the endoscopic biopsy demonstrated IMC in one patient who opted for surgical treatment.
In 49% of the 104 patients in whom an EMR was performed, the final pathologic assessment was discordant when compared with pretreatment biopsies. Upstaging was observed in 21.1% of patients (N=22) and down-staging occurred in 27.9% of patients (N=29). Final histopathology staging of all patients after EMR or esophagectomy is shown in Table 2 according to the Vienna Classification (10).
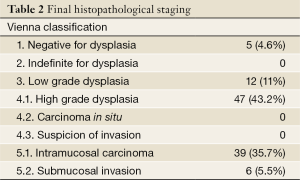
Full table
A total of 99 macroscopically visible lesions (VL) were recognized in 81 patients (74.3%), nine patients had two concurrent lesions and five patients had three concurrent VL.
EUS Findings
Table 3 shows the information from reviewed EUS reports. TNM staging was reported in 14 of 109 EUS procedures: 4 patients were staged as T1aN0Mx and 10 as T1bNxMx. In the remaining 95 patients, the EUS report documented that there was no evidence of invasive or distant disease.
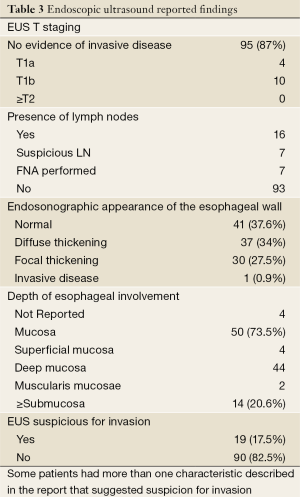
Full table
Lymph nodes (LN) were identified in 16 patients. According to the previously mentioned endosonographic criteria (size >10 mm, round shape, sharp borders and hypoechoic/heterogeneous aspect), a suspicion of malignancy was present in seven patients. FNA was performed in each of these 7 cases and none of the cytological exams revealed presence of tumor cells.
EUS exams reported diffuse or focal thickening of the esophageal wall in 68 patients. Depth of these esophageal findings was not recorded in 4 patients, involved the submucosa or beyond in 14 patients (20.6%), and were limited to the mucosal layer (superficial mucosa, deep mucosa and muscularis mucosae) in 50 cases (73.5%). Of those with thickening limited to the mucosal layer, 3 cases had no dysplasia, 44 had neoplasia confined to the mucosa (5 LGD, 23 HGD and 16 IMC), and 3 cases had submucosal involvement (6%). EMR or surgery confirmed invasive neoplasia only in 3 (21.4%) among the 14 patients with diffuse or focal esophageal wall thickening involving the submucosa noted on EUS; the remaining 11 patients (78.6%) had neoplasia limited to the mucosa (9 IMC, 2 HGD) (Table 4).

Full table
EUS reports were classified as having no findings suspicious for invasion in 90 of 109 patients (82.6%), final staging in this group was: ≤ T1a =87 and ≥ T1b =3 (3.4%). Only 19 patients (17.4%) were categorized as having EUS findings suspicious for invasion, final staging was: ≤ T1a =16 (HGD =4 and IMC =12) and ≥ T1b =3. The global sensitivity, specificity, accuracy, positive predictive value (PPV) and negative predictive value (NPV) of the EUS in detection of invasion are shown in Table 5.
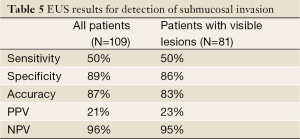
Full table
The presence of VL between patients with EUS findings suspicious for invasion and those with negative esophageal EUS findings were not statistically different [16/19 (84%) vs. 65/90 (72%) P=0.42]. Three (16%) of the 19 patients with EUS findings suspicious for invasion had flat BE, and none of these three had evidence of invasion on pathology. EUS findings were considered suspicious in 3 out of 9 patients with a predominant protruding lesion (types 0-Is and 0-Ip); 6 out of 38 patients with a slightly elevated lesion (only 0-IIa); 2 out of 8 with a flat lesion (only 0-IIb); none of 4 with concurrent elevated and flat lesions (concurrent 0-IIa and 0-IIb) and 5 out of 22 with any evidence of depressed lesions (0-IIc or 0-III or any depressed component in any lesion). However, there were no cases of SMI in any patients with only 0-IIa or 0-IIb lesions. Moreover, the accuracy of EUS for SMI of patients with a predominant protruding lesion was not better than the global accuracy of 87%.
Of the 86 patients with HGD or IMC as diagnosed by histologic specimens provided by EMR or surgery, sixteen (18.6%) had the pre-therapeutic EUS findings suspicious for invasion. Of the 6 patients with submucosal involvement in pathology analysis (≥T1sm1), only 3 (50%) had EUS findings suspicious for invasion before treatment. Patients with EUS findings suspicious for invasion more commonly had submucosal involvement in the EMR/surgery samples compared to those with other EUS findings [3/19 (15.8%) vs. 3/90 (3.33%) P=0.06], but the observed difference was not statistically significant. Forty-one patients had unremarkable EUS findings in the entire esophagus; in all of them the EMR confirmed absence of invasive disease and highest staging was IMC in 14 (34%).
Incidental findings unrelated to the main indication for the EUS were diagnosed in 11 of the total 109 patients (10%). EUS examinations revealed gallbladder stones in 5 cases, pancreatic lesions in 4 (one tumor consistent with adenocarcinoma after FNA and three cystic lesions), and one liver cyst and one mediastinal mass consistent with a carcinoid tumor.
Discussion
The newly developed endoscopic treatments for early Barrett’s neoplasia offer curative therapy with minimal invasiveness to patients with cancer limited to the mucosal layer. The risk of nodal involvement in early esophageal cancer confined to the mucosa (T1a) ranges between 0% and 3%, and when the lesion extends into the submucosal layer (T1b) this risk approaches up to as high as 30% (9). Because of the radical differences in the therapeutic approach to cancer confined to the mucosa vs. invasive cancer it is essential to provide accurate tumor (T) and node (N) staging in the selection of patients with early Barrett’s neoplasia for curative endoscopic therapy. The critical depth assessment of early Barrett’s neoplasia is to distinguish T1b from T1a lesions; the latter can be successfully treated with endoscopic therapy, while the former requires surgical resection (6). While EUS is considered the best tool for T and N staging of esophageal cancer (11-15), its performance in early Barrett’s neoplasia is suboptimal for tumor depth assessment.
Conventional EUS, with frequencies between 7 MHz and 12 MHz, displays the esophageal wall in five different layers and the muscularis mucosae is not visualized as a separate layer (3,16-19). With high frequency echo-endoscopes and high frequency mini-probes (HFP) (20-30 MHz) the mucosa is seen in four different layers and the muscularis mucosae can be assessed separately (3,17-20). The only prospective comparative study published to date (21) showed that the use of HFP is significantly better than conventional radial EUS in the T staging [P<0.0001]; however, the accuracy is low with both techniques (64% and 49% respectively).
The reported accuracy rate in the staging of early esophageal cancer are still disappointing and heterogeneous (4,21-28), and widely ranges from the 85% reported by Larghi et al. (21) to 79.6% from May et al. (22) and to the 69% reported by Pech et al. (24).
In the present study, the accuracy of identifying submucosal invasion was consistent with previously published data and emphasizes that the role of EUS in the pretreatment management of patients with early Barrett’s neoplasia is still controversial. EUS led to an overstaging in most of patients, in 14 with endosonographic diffuse or focal thickening of the esophageal wall involving the submucosa, EMR revealed neoplasia confined to the mucosal layer in up to 78.6%. All of these cases could have been potentially treated by endoscopic therapy, avoiding other more invasive treatments with associated higher mortality and morbidity rates. These results also highlight the role of EMR as a diagnostic and staging tool, providing an accurate evaluation of the resection margins, submucosal involvement, and risk factors for presence of lymph node metastasis. In our cohort, analysis of EMR specimens changed the final staging in 49% of 104 patients, which is consistent with published data (28-30) and dramatically changes the clinical management of these patients. Upstaging was observed in 21.1% (N=22) and downstaging in 27.9% (N=29).
The pattern of invasion and the risk of lymph node metastasis in early Barrett’s adenocarcinoma are clearly related to the depth of tumor infiltration in the esophageal wall (31,32). A recent published review of 805 endoscopic resections from 472 patients shows that the depth of invasion correlates with differentiation grade, lymphatic vessels involvement and venous involvement, all of them well established risk factors for developing lymph node metastasis (33). Several studies assess the correlation between tumor infiltration and prevalence of lymph node metastasis (34-47). In case of high grade intraepithelial neoplasia (high grade dysplasia not beyond the basal membrane) the risk of lymph node metastasis is absent. For T1a tumors (not beyond muscularis mucosae) the reported rates of lymphatic involvement are <1%. Tumors invading the submucosal layer (T1b) had a prevalence of lymph node metastasis between 20% and 30%. Our study shows that patients with neoplasia invading the submucosal layer in final staging are not more likely to have findings suspicious for invasion in the EUS exam; only 50% of patients with ≥ Tb tumors had a previous suspicion for invasion on EUS.
The presence of malignant lymph nodes and/or submucosal invasion radically changes the therapeutic approach of early Barrett’s neoplasia. Therefore, in this study, all EUS exams were considered to have findings suspicious for invasion based on the presence of these two conditions (EUS stage ≥T1bNxMx and/or thickening of the esophageal wall involving the submucosal layer and/or presence of suspicious lymph nodes).
Up to 82.5% of the EUS exams were considered as findings not suspicious for invasion according to the aforementioned criteria. Of all 19 (17.5%) patients with EUS findings suspicious for invasion, only 3 (15.8%) had submucosal involvement on the final pathology. The remaining 84.2% had neoplasia limited to the mucosal layer (≤T1a) that could be successfully treated with endoscopic approaches.
Despite 17% of patients with findings suspicious for invasion in the EUS exam, its clinical impact in the treatment algorithm of early Barrett’s neoplasia is negligible. 84% of them had no evidence of invasion and should be considered as false positives; the true positive rate of findings suspicious for invasion on EUS was as low as 16%. Thus, even in patients with early Barrett’s neoplasia and findings suspicious for invasion, EUS did not provide any additional information for making decision of treatment for patients at this center.
Surveillance endoscopy with high-resolution endoscopy (HRE) is the most effective tool to detect premalignant and malignant lesions of the GI tract in an early stage. In Barrett’s patients, the endoscopic appearance of any superficial lesion according to the Paris Classification (9,48), helps to predict the presence of submucosal invasion, that is clearly related with the risk of nodal metastasis (49). Two prospective studies did not demonstrate statistically significant differences between EUS and HRE for staging of early gastric (50) and early esophageal cancer (22), but in Tsm1 tumors the reported accuracy of both techniques is yet far to be satisfactory and up to 40% of cases with submucosal infiltration were not identified with combination of HRE and EUS (22).
The limitations of EUS for an accurate diagnosis of early Barrett’s cancer seem to be higher in flat and depressed lesions according to the published data (21). In our study, no statistically significant difference in proportion of patients with EUS findings suspicious for invasion regarding the presence of any visible lesion was noted. When the type of lesion was analyzed, no statistically significant association between significant EUS findings and flat lesions (type 0-IIb) was found.
Our results are consistent with the most recently published studies about this topic. Pech et al. (51) reported an unsatisfactory accuracy rate of 74% for T stage and 73% for N stage when comparing EUS staging before surgery with esophagectomy staging (n=179). T2 cancers are the most frequently overstaged by EUS, leading in a significant impact on making treatment decisions.
Similarly to our data, Thomas et al. (52) reported that the role of EUS in the pretherapeutic algorithm for early Barrett’s neoplasia should be reconsidered with submucosal invasion detected only in 26% of patients (n=50). The value of EUS is even more limited in patients with flat VL (0-IIb), where all of lesions are confined to the mucosa.
In the same direction, a recent retrospective analysis of 131 patients with early esophageal cancer performed by the Amsterdam group (53) concluded that EUS exam has no clinical impact on the decision making for treatment. 24% of the 105 patients with unremarkable EUS findings underwent surgery after EMR due to submucosal involvement, positive resection margins, lymphovascular invasion or poor differentiation grade. In the other hand, 38% of the 26 patients with suspected submucosal invasion or LNM according to the EUS exams were successfully treated by endoscopic approach.
A recent review established a global incidence of incidental findings (in radiological tests of 23.6%, which were detected in higher frequencies when CT scan was performed. However, none of the included studies in this review had reported data from EUS exams (54). In this series, 10% (n=11) of patients had an additional diagnosis due to the EUS exam; in 6 of the 11 patients, these incidental findings were considered as significant according to the need for further investigations, treatment or follow up (4 pancreatic lesions and 1 mediastinal mass). The only study published to date, which reports incidental finding rates on EUS (55), found an overall 38.5% incidence of additional ancillary diagnoses in 239 consecutive EUS exams performed for a variety of indications. Of these incidentally found conditions, 11.3% were considered clinically significant. These findings raise the question if a complete endosonographic exploration should be performed in every patient.
There are several limitations to our study, including a retrospective design based on the information provided by clinical reports from a single center. This study presents a markedly low rate of patients with TNM staging reported on the final EUS diagnosis. All the information related to the depth of tumor was collected from the descriptions. The sample size of some subgroups was small, mainly patients with ≥ T1b tumors and lymph node involvement. One explanation of the low prevalence of these two conditions in our cohort is that we only enrolled patients with superficial neoplasia; the patients who are more likely to have advanced disease with obvious masses were excluded.
Conclusions
Most patients referred for consideration of endoscopic or surgical treatment of early BE neoplasia have unremarkable findings on EUS exam. The assessment of the invasion depth of early Barrett’s neoplasia based only in the EUS findings, leads to an overstaging in most of patients with a false positive rate for diagnosis of submucosal invasion up to 84%. Given the high false positives rate for submucosal invasion and most of patients with suspicion of invasive disease according to the EUS findings had lesions limited to the mucosa, EUS has limited value in the pre-therapeutic algorithm of patients with early Barrett’s neoplasia and has negligible impact in making decisions for therapy. EUS in the pre-therapeutic evaluation of early Barrett’s neoplasia does continue to have a role to rule out the presence of lymph node metastasis in cases with known cancer or suspected advanced pathology in settings of visible lesions.
Acknowledgements
This work was partially supported by a grant from the Consejería de Salud y Servicios Sanitarios of the Principality of Asturias (Asturias, Spain).
Disclosure: The authors declare no conflict of interest.
References
- Wang KK, Sampliner REPractice Parameters Committee of the American College of Gastroenterology. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett’s esophagus. Am J Gastroenterol 2008;103:788-97. [PubMed]
- Attila T, Faigel DO. Role of endoscopic ultrasound in superficial esophageal cancer. Dis Esophagus 2009;22:104-12. [PubMed]
- Bergman JJ. The endoscopic diagnosis and staging of oesophageal adenocarcinoma. Best Pract Res Clin Gastroenterol 2006;20:843-66. [PubMed]
- Chemaly M, Scalone O, Durivage G, et al. Miniprobe EUS in the pretherapeutic assessment of early esophageal neoplasia. Endoscopy 2008;40:2-6. [PubMed]
- Hünerbein M, Ulmer C, Handke T, et al. Endosonography of upper gastrointestinal tract cancer on demand using miniprobes or endoscopic ultrasound. Surg Endosc 2003;17:615-9. [PubMed]
- Adrain AL, Ter HC, Cassidy MJ, et al. High-resolution endoluminal sonography is a sensitive modality for the identification of Barrett’s metaplasia. Gastrointest Endosc 1997;46:147-51. [PubMed]
- Waxman I, Raju GS, Critchlow J, et al. High-frequency probe ultrasonography has limited accuracy for detecting invasive adenocarcinoma in patients with Barrett’s esophagus and high-grade dysplasia or intramucosal carcinoma: a case series. Am J Gastroenterol 2006;101:1773-9. [PubMed]
- Thomas T, Gilbert D, Kaye PV, et al. High-resolution endoscopy and endoscopic ultrasound for evaluation of early neoplasia in Barrett’s esophagus. Surg Endosc 2010;24:1110-6. [PubMed]
- The Paris Endoscopic Classification of Superficial Neoplastic Lesions. Esophagus, stomach, and colon. Gastrointest Endosc 2003;58:S3-S43. [PubMed]
- Schlemper RJ, Riddell RH, Kato Y, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut 2000;47:251-5. [PubMed]
- van Vliet EP, Heijenbrok-Kal MH, Hunink MG, et al. Staging investigations for oesophageal cancer: a meta-analysis. Br J Cancer 2008;98:547-57. [PubMed]
- Botet JF, Lightdale CJ, Zauber AG, et al. Preoperative staging of esophageal cancer: comparison of endoscopic US and dynamic CT. Radiology 1991;181:419-25. [PubMed]
- Korst RJ, Altorki NK. Imaging for esophageal tumors. Thorac Surg Clin 2004;14:61-9. [PubMed]
- Choi J, Kim SG, Kim JS, et al. Comparison of endoscopic ultrasonography (EUS), positron emission tomography (PET), and computed tomography (CT) in the preoperative locoregional staging of resectable esophageal cancer. Surg Endosc 2010;24:1380-6. [PubMed]
- Van Dam J. Endosonographic evaluation of the patient with esophageal cancer. Chest 1997;112:184S-190S. [PubMed]
- Rösch T. Endosonographic staging of esophageal cancer: a review of literature results. Gastrointest Endosc Clin N Am 1995;5:537-47. [PubMed]
- Dunn J, Lovat L. The role of endoscopic ultrasonography in Barrett’s esophagus and early esophageal cancer. Tech Gastrointest Endosc 2010;12:12-7.
- Savoy AD, Wallace MB. EUS in the management of the patient with dysplasia in Barrett’s esophagus. J Clin Gastroenterol 2005;39:263-7. [PubMed]
- Shami VM, Villaverde A, Stearns L, et al. Clinical impact of conventional endosonography and endoscopic ultrasound-guided fine-needle aspiration in the assessment of patients with Barrett’s esophagus and high-grade dysplasia or intramucosal carcinoma who have been referred for endoscopic ablation therapy. Endoscopy 2006;38:157-61. [PubMed]
- Rampado S, Bocus P, Battaglia G, et al. Endoscopic ultrasound: accuracy in staging superficial carcinomas of the esophagus. Ann Thorac Surg 2008;85:251-6. [PubMed]
- Larghi A, Lightdale CJ, Memeo L, et al. EUS followed by EMR for staging of high-grade dysplasia and early cancer in Barrett’s esophagus. Gastrointest Endosc 2005;62:16-23. [PubMed]
- May A, Günter E, Roth F, et al. Accuracy of staging in early oesophageal cancer using high resolution endoscopy and high resolution endosonography: a comparative, prospective, and blinded trial. Gut 2004;53:634-40. [PubMed]
- Pech O, May A, Günter E, et al. The impact of endoscopic ultrasound and computed tomography on the TNM staging of early cancer in Barrett’s esophagus. Am J Gastroenterol 2006;101:2223-9. [PubMed]
- Pech O, Günter E, Dusemund F, et al. Value of high-frequency miniprobes and conventional radial endoscopic ultrasound in the staging of early Barrett’s carcinoma. Endoscopy 2010;42:98-103. [PubMed]
- Murata Y, Suzuki S, Ohta M, et al. Small ultrasonic probes for determination of the depth of superficial esophageal cancer. Gastrointest Endosc 1996;44:23-8. [PubMed]
- Yanai H, Yoshida T, Harada T, et al. Endoscopic ultrasonography of superficial esophageal cancers using a thin ultrasound probe system equipped with switchable radial and linear scanning modes. Gastrointest Endosc 1996;44:578-82. [PubMed]
- Hasegawa N, Niwa Y, Arisawa T, et al. Preoperative staging of superficial esophageal carcinoma: comparison of an ultrasound probe and standard endoscopic ultrasonography. Gastrointest Endosc 1996;44:388-93. [PubMed]
- Hull MJ, Mino-Kenudson M, Nishioka NS, et al. Endoscopic mucosal resection: an improved diagnostic procedure for early gastroesophageal epithelial neoplasms. Am J Surg Pathol 2006;30:114-8. [PubMed]
- Chennat J, Konda VJ, Ross AS, et al. Complete Barrett’s eradication endoscopic mucosal resection: an effective treatment modality for high-grade dysplasia and intramucosal carcinoma--an American single-center experience. Am J Gastroenterol 2009;104:2684-92. [PubMed]
- Moss A, Bourke MJ, Hourigan LF, et al. Endoscopic resection for Barrett’s high-grade dysplasia and early esophageal adenocarcinoma: an essential staging procedure with long-term therapeutic benefit. Am J Gastroenterol 2010;105:1276-83. [PubMed]
- Feith M, Stein HJ, Siewert JR. Pattern of lymphatic spread of Barrett’s cancer. World J Surg 2003;27:1052-7. [PubMed]
- Stolte M, Kirtil T, Oellig F, et al. The pattern of invasion of early carcinomas in Barrett’s esophagus is dependent on the depth of infiltration. Pathol Res Pract 2010;206:300-4. [PubMed]
- Zemler B, May A, Ell C, et al. Early Barrett’s carcinoma: the depth of infiltration of the tumour correlates with the degree of differentiation, the incidence of lymphatic vessel and venous invasion. Virchows Arch 2010;456:609-14. [PubMed]
- Buskens CJ, Westerterp M, Lagarde SM, et al. Prediction of appropriateness of local endoscopic treatment for high-grade dysplasia and early adenocarcinoma by EUS and histopathologic features. Gastrointest Endosc 2004;60:703-10. [PubMed]
- Westerterp M, Koppert LB, Buskens CJ, et al. Outcome of surgical treatment for early adenocarcinoma of the esophagus or gastro-esophageal junction. Virchows Arch 2005;446:497-504. [PubMed]
- Badreddine RJ, Prasad GA, Lewis JT, et al. Depth of submucosal invasion does not predict lymph node metastasis and survival of patients with esophageal carcinoma. Clin Gastroenterol Hepatol 2010;8:248-53. [PubMed]
- Bollschweiler E, Baldus SE, Schröder W, et al. High rate of lymph-node metastasis in submucosal esophageal squamous-cell carcinomas and adenocarcinomas. Endoscopy 2006;38:149-56. [PubMed]
- Sepesi B, Watson TJ, Zhou D, et al. Are endoscopic therapies appropriate for superficial submucosal esophageal adenocarcinoma? An analysis of esophagectomy specimens. J Am Coll Surg 2010;210:418-27. [PubMed]
- Liu L, Hofstetter WL, Rashid A, et al. Significance of the depth of tumor invasion and lymph node metastasis in superficially invasive (T1) esophageal adenocarcinoma. Am J Surg Pathol 2005;29:1079-85. [PubMed]
- Ancona E, Rampado S, Cassaro M, et al. Prediction of lymph node status in superficial esophageal carcinoma. Ann Surg Oncol 2008;15:3278-88. [PubMed]
- Hölscher AH, Bollschweiler E, Schneider PM, et al. Early adenocarcinoma in Barrett’s oesophagus. Br J Surg 1997;84:1470-3. [PubMed]
- Rice TW, Zuccaro G Jr, Adelstein DJ, et al. Esophageal carcinoma: depth of tumor invasion is predictive of regional lymph node status. Ann Thorac Surg 1998;65:787-92. [PubMed]
- Nigro JJ, Hagen JA, DeMeester TR, et al. Prevalence and location of nodal metastases in distal esophageal adenocarcinoma confined to the wall: implications for therapy. J Thorac Cardiovasc Surg 1999;117:16-23; discussion 23-5. [PubMed]
- Stein HJ, Feith M, Mueller J, et al. Limited resection for early adenocarcinoma in Barrett’s esophagus. Ann Surg 2000;232:733-42. [PubMed]
- van Sandick JW, van Lanschot JJ, ten Kate FJ, et al. Pathology of early invasive adenocarcinoma of the esophagus or esophagogastric junction: implications for therapeutic decision making. Cancer 2000;88:2429-37. [PubMed]
- Stein HJ, Feith M, Bruecher BL, et al. Early esophageal cancer: pattern of lymphatic spread and prognostic factors for long-term survival after surgical resection. Ann Surg 2005;242:566-73; discussion 573-5. [PubMed]
- Manner H, May A, Pech O, et al. Early Barrett’s carcinoma with “low-risk” submucosal invasion: long-term results of endoscopic resection with a curative intent. Am J Gastroenterol 2008;103:2589-97. [PubMed]
- Endoscopic Classification Review Group. Update on the paris classification of superficial neoplastic lesions in the digestive tract. Endoscopy 2005;37:570-8. [PubMed]
- Pech O, Gossner L, Manner H, et al. Prospective evaluation of the macroscopic types and location of early Barrett’s neoplasia in 380 lesions. Endoscopy 2007;39:588-93. [PubMed]
- Yanai H, Noguchi T, Mizumachi S, et al. A blind comparison of the effectiveness of endoscopic ultrasonography and endoscopy in staging early gastric cancer. Gut 1999;44:361-5. [PubMed]
- Pech O, Günter E, Dusemund F, et al. Accuracy of endoscopic ultrasound in preoperative staging of esophageal cancer: results from a referral center for early esophageal cancer. Endoscopy 2010;42:456-61. [PubMed]
- Thomas T, Gilbert D, Kaye PV, et al. High-resolution endoscopy and endoscopic ultrasound for evaluation of early neoplasia in Barrett’s esophagus. Surg Endosc 2010;24:1110-6. [PubMed]
- Pouw RE, Heldoorn N, Herrero LA, et al. Do we still need EUS in the workup of patients with early esophageal neoplasia? A retrospective analysis of 131 cases. Gastrointest Endosc 2011;73:662-8. [PubMed]
- Lumbreras B, Donat L, Hernández-Aguado I. Incidental findings in imaging diagnostic tests: a systematic review. Br J Radiol 2010;83:276-89. [PubMed]
- Vila JJ, Jiménez FJ, Irisarri R, et al. Prospective observational study of the incidental findings on endoscopic ultrasonography: should a complete exploration always be performed? Scand J Gastroenterol 2009;44:1139-45. [PubMed]


