Hepatic imaging response to radioembolization with yttrium-90-labeled resin microspheres for tumor progression during systemic chemotherapy in patients with colorectal liver metastases
Background
The liver is a common site of metastasis among patients with colorectal cancer (mCRC) (1,2). Surgical resection, if possible, remains standard treatment for these tumors. However, several factors, including anatomical location of the tumor, extent of hepatic metastases, inadequate hepatic functional reserve, and comorbidities result in some 75-90% of patients being ineligible for surgical treatment (3). For these patients, local or regional therapy options are available.
Radioembolization (RE) with ytrium-90-labeled (90Y) microspheres is a form of brachytherapy that exhibits anti-tumor activity via radiation damage from locally implanted microspheres (4). These microspheres are 30 microns in diameter, and are administered via hepatic vasculature so that they permanently implant in the terminal arterioles of hepatic tumors. Normal liver parenchyma adjacent to the tumor is spared injury because the mean penetration of beta radiation is 2.5 mm (and no greater than 11 mm) (4). In clinical studies, yttrium-90-labeled resin microspheres (90Y-RE) has been combined with 5-fluorouracil, leucovorin, and oxaliplatin or irinotecan (i.e., FOLFOX or FOLFIRI) during first- or second-line chemotherapy, or administered alone or in combination with 5-fluorouracil in the refractory setting (5-8). Compared with systemic chemotherapy alone, clinical trials have demonstrated improvements in progression-free survival (PFS), overall survival (OS) and objective response rates with the addition of 90Y-RE, even among heavily pre-treated patients (6-12). Despite the success of 90Y-RE in prospective clinical trials, frequent questions still arise during tumor boards and patient consultations about the typical response to treatment and the reliability of Response Evaluation Criteria in Solid Tumors (RECIST) (13-15). Moreover, a recent review found that the time to response measured on CT varied widely between studies from 1.5 to 6 months (16); although the majority of studies, including a study by Kennedy et al. [2006] (17), found that the optimum time to response is at approximately 3 months post-procedure. The purpose of this retrospective study was to assess the imaging response at 3 months in patients with hepatic metastases secondary to colorectal cancer (CRC) who were treated with 90Y-RE in community and academic cancer centers in the United States. Data from the primary analyses in the overall cohort are published elsewhere (18).
Methods
Selection of institutions and patient cases
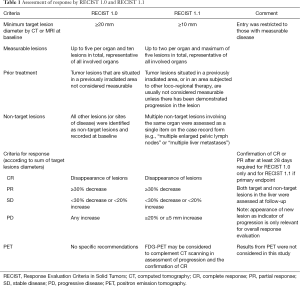
Eleven of the 15 invited RE centers in the United States participated in a retrospective study of mCRC liver metastases outcomes after RE (MORE). Institutional review boards granted exemptions for each participating site prior to the start of data collection. Data were collected from source documentation by an independent contract research organization for all patients with a diagnosis of mCRC who were treated with 90Y-resin microspheres (SIR-Spheres®; Sirtex Medical, Sydney, Australia) between July 2002 and December 2011 and had at least one follow-up visit. Patient identifiers were replaced with a unique study number. This imaging response report was conducted in a sub-cohort of patients from the MORE study of only those patients from nine centers with radiologic studies which meet our strict criteria of pre-treatment and post treatment time intervals. These were (I) within 30 days prior to 90Y-RE, and (II) at 90 days (±30 days) post 90Y-RE. Only these studies were analyzed via independent central imaging review and comprise the dataset for this report. A board-certified radiologist expert in post 90Y-RE treated patients systematically reviewed abdominal computed tomography (CT) images (portal venous phase) collected at baseline and 3 months following the first 90Y-RE procedure. Response to treatment was assessed using the RECIST versions 1.0 (19) and 1.1 (20), based on a maximum of five and two target lesions respectively (Table 1). Peri-tumoral edema and necrosis (known artifacts which can impact interpretation of response) were also documented for each lesion.
Full table
As per the published guidance at the time of the study (8,21-24), 90Y-RE was considered for those patients with advanced liver-dominant mCRC who were not suitable for surgery, ablation or systemic therapy, and had progressed or become intolerant to at least one line of systemic therapy (Table 2). During the pre-treatment work-up, patients were excluded from RE if there was evidence of any uncorrectable flow to non-target sites (e.g., gastrointestinal tract or other extra-hepatic organs) observed on angiography or Technetium-99m macroaggregated albumin (99mTc-MAA) scans. Some patients, under exceptional circumstances and with informed consent, were treated outside the criteria outlined above based on the clinical judgment of the treating physicians. The protocol for treatment is reported elsewhere for the administration of 90Y-resin microspheres within a single session or over multiple sessions (e.g., using a sequential lobar approach for bilobar liver metastases) (22). The body surface area methodology was mainly used in the activity calculations for 90Y.

Full table
Statistical methodology
This study tested no formal hypotheses. Descriptive statistics were conducted using SAS version 9.2 XP Pro statistical analyses software (SAS Institute Inc., Cary NC, USA) to summarize patient characteristics. Estimates of OS were computed by response to treatment [partial response (PR) versus stable (SD) or progressive disease (PD)] and the activity delivered (with the first RE procedure and overall) using Kaplan-Meier product-limit method (25).
Results
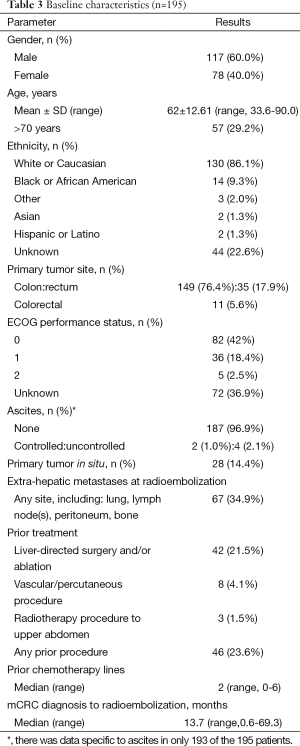
A total of 195 patients (male, 60%; Caucasian, 67%) received a median of 2 (range, 0-6) lines of chemotherapy prior to 90Y-RE. Patient characteristics are summarized in Table 3. Median tumor/liver ratio at the start of 90Y-RE was 15% [interquartile range (IQR): 24%]. Median 90Y activity administered was 1.18 GBq (IQR: 0.59).
Full table
Response to treatment and OS
Best response and response at 3-month follow-up by RECIST 1.0 and 1.1 are shown in Table 4. Three-month responses were assessed in 131 patients, with a median time to follow-up of 82 days (IQR: 34). The median time to best response was 70 days (IQR: 55). This difference in median time to responses is due to the range of times accepted as the 3-month evaluation scan which included studies at 90±30 days. This was necessary as patients were not entered on a prospective trial and thus imaging studies were completed in a less strict time course which was intended to be 3 months after 90Y-RE. There was good agreement between responses assessed by RECIST 1.0 and 1.1, for best response {kappa =0.96, [95% confidence interval (CI): 0.855-0.956]} and for the response at 3 months [kappa =0.915, (95% CI: 0.856-0.975)]. No significant differences in baseline characteristics for responders and non-responders were evident (P>0.05).

Full table
In patients for whom 3-month follow-up imaging was evaluated, necrosis and peri-tumoral edema (by RECIST 1.0) was documented in 48.1% and 32.8% of patients, respectively. Both necrosis and peri-tumoral edema were observed in 57.3% of patients. By RECIST 1.1, necrosis and peri-tumoral edema were observed in 41.2% and 29.8%, respectively, with both necrosis and peri-tumoral edema documented in 50.4% of patients.
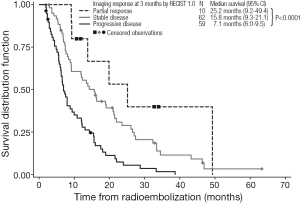
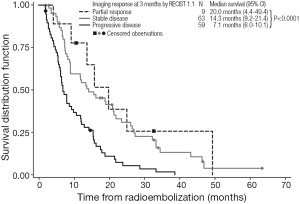
Kaplan-Meier estimates of OS by response to treatment by RECIST 1.0 and RECIST 1.1 are shown in Figures 1,2, respectively. For both RECIST 1.0 and 1.1, response at 3 months significantly predicted survival (P<0.0001).
Relationship between activity delivered and response or OS
Further analyses found that there was no relationship between the total activity of 90Y delivered and the response to RE, when assessed by either RECIST 1.0 (P=0.487) or RECIST 1.1 (P=0.710). However, patients who received a lower activity (<1 vs. ≥1 GBq) with the first RE procedure had a significantly prolonged survival: 15.7 (95% CI: 12.1-21.6) vs. 9.2 (95% CI: 8.1-11.2) months; P=0.006 (LogRank); as well as patients who received a lower activity (<1 vs. ≥1 GBq) overall: 17.4 (95% CI: 12.1-28.9) vs. 9.3 (95% CI: 8.2-12.1) months; P=0.011 (LogRank). However univariate assessment found no correlation across all activities delivered and OS (P=0.474).
Discussion
As a treatment for patients with hepatic metastases secondary to CRC, the addition of 90Y-RE to systemic therapy has been shown to improve PFS, OS and response rate compared with chemotherapy alone in prospective clinical trials (6,9,10,12). This retrospective study sought to assess the imaging response in patients treated with 90Y-RE in both community and academic cancer centers. Among the 195 patients included, disease control (PR or SD) was evident in 62.1% and 63.1% by RECIST 1.0 and 1.1, respectively, with a high rate of agreement between the two assessment methods. These results compare favorably with recent trials with newer therapies in mCRC (such as regorafenib), which achieved a disease control rate of 41.0% (PR: 1%; SD: 40%) in a similar cohort of chemorefractory patients (26). However, this study emphasizes the need for cautious interpretation of radiological response at 3 months with RE, with a significant proportion of patients’ images demonstrating necrosis and/or peri-tumoral edema, which can lead to either underestimation of response or overestimation of progression. This reflects the findings of other research groups evaluating the early response with either 90Y glass (27,28) or resin microspheres (29), especially when assessing the response to treatment at less than 3 months after the procedure (16).
As summarized in Table 5, contemporary studies reporting radiologic response after RE with either resin or glass 90Y microspheres compare closely with the current study. When grouped by line of therapy—first-line (8-10,24) second or third-line (11,45) chemotherapy refractory disease (5,6,17,30-44) and mixed first-line through chemotherapy refractory disease, there is a trend toward higher response earlier in the disease course.
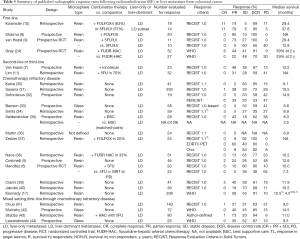
Full table
Despite these caveats, radiological response to 90Y-RE at 3 months appears to predict longer-term prognosis in the management of liver-dominant mCRC (5,43). We found that assessments of OS showed median survivals of 25.2 months for partial responders (by RECIST 1.0 at 3 months), with significantly shorter median survivals for patients with stable disease (15.8 months) or disease progression (7.1 months). These trends were similar when either RECIST 1.0 or RECIST 1.1 assessed responses at 3 months. Notably RECIST 1.1 requires the assessment of only two target lesions per organ (not less than 5 mm in size) instead of five as used in RECIST 1.0 (46); however, RECIST 1.1 has the advantage in that it may enable the more accurate diagnosis of progression (specified as an increase of 20% or more in the sum of the longest diameters of the target lesions), because it eliminates the interpretation of small increases in the tumor size as a significant increase in tumor burden (46). Although not assessed in this study, RECIST 1.1 also allows the findings from positron emission tomography (PET) to be considered in support CT findings, for PD and confirmation of complete response (CR) (16). Several studies assessing the prognostic value of response rate to 90Y-RE have assessed CT findings in conjunction with tumor markers such as carcinoembryonic antigen (CEA) (38,43).
Beyond the measurement of anatomical changes in tumors, the development of functional imaging techniques including diffusion-weighted magnetic resonance imaging (DW-MRI) for hepatocellular carcinoma (HCC) (47-49) and mCRC (50-52), gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging (Gd-EOB-DTPA) in HCC and mCRC (52,53), and PET for liver metastases (14,27,29,44,54,55) have allowed for the earlier (between 6-8 weeks post-procedure) and/or more sensitive assessment of treatment response compared with CT using RECIST (29,56). More recently, changes in metabolic volume and total lesion glycolytic rate as measured by fluorine-18 fluorodeoxyglucose (18F-FDG) PET in response to 90Y-RE has shown to be predictive of survival (57) while changes in maximum standardized uptake value (SUVmax) on 18F-FDG PET have shown to be predictive of PFS (37). As imaging techniques evolve, the utility of pretreatment imaging (such as contrast-enhanced CT perfusion of liver metastases) in predicting potential responders and survival following 90Y-RE prior presents an intriguing new development (58); although further validation of these imaging techniques is still needed before they are adopted in clinical practice. Currently, several multicenter phase III trials with 90Y-RE are ongoing including SORAMIC which is evaluating Gd-EOB-DTPA-MRI in HCC, while the SIRFLOX and the FOXFIRE studies in mCRC are evaluating the response using RECIST 1.0 and modified RECIST, respectively.
The study also found that patients who received a low activity (<1 GBq of 90Y), probably reflecting a lower disease burden in the liver, had a significantly longer survival than patients who were required higher activities of 90Y. Overall, the activity delivered was not predictive of response at 3 months when measured by RECIST 1.0 or RECIST 1.1 in this cohort of patients.
The main limitation of this study is the retrospective nature of analyses. The MORE study permitted a broader range of patients than would otherwise be included within conventional clinical trials with chemotherapy (from some who received 90Y-RE as a first-line therapy to others who received 90Y-RE in the chemorefractory setting after three or more prior lines of chemotherapy). Nevertheless, careful guidance in the selection of patients based on published consensus from the RE Brachytherapy Oncology Consortium (REBOC) and other earlier reviews (21-23) allowed for the inclusion of patients of a similar stage (with liver-dominant disease and an ECOG performance status 0-1). This homogeneity was important to our findings since baseline factors such as extrahepatic disease as well as ECOG performance status are also important predictors of survival following 90Y-RE (10,38).
In conclusion, while this study is not without the limitations common to all retrospective studies, it provides a unique assessment of tumor response after 90Y-RE in patients treated in both community and academic cancer centers. Even in these unselected patients, the benefit of 90Y-RE for patients with unresectable hepatic metastases secondary to CRC is evident.
Acknowledgements
We would like to thank Mark Van Buskirk for his outstanding statistical work and advice; and Rae Hobbs for her editorial assistance.
Funding: This was an investigator-initiated study funded by Sirtex Medical Limited, Sydney, Australia through an educational grant awarded to Dr. Kennedy, Sarah Cannon Research Institute.
Footnote
Conflicts of Interest: DM Coldwell is a consultant to Sirtex Medical; M Cohn, A Drooz, FM Moeslein, CW Nutting, SG Putnam 3rd, SC Rose, EA Wang are proctors for Sirtex Medical; MA Savin is a speaker for BSD Medical; E Ehrenwald, S Kanani, S Schirm have nothing to declare.
Financial Disclosure: AS Kennedy, D Ball, NK Sharma received grants for clinical trials from Sirtex Medical.
Prior presentations: AS Kennedy et al. GI ASCO 2013.
References
- Manfredi S, Lepage C, Hatem C, et al. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg 2006;244:254-9. [PubMed]
- McMillan DC, McArdle CS. Epidemiology of colorectal liver metastases. Surg Oncol 2007;16:3-5. [PubMed]
- Young AL, Adair R, Culverwell A, et al. Variation in referral practice for patients with colorectal cancer liver metastases. Br J Surg 2013;100:1627-32. [PubMed]
- Kennedy AS, Nutting C, Coldwell D, et al. Pathologic response and microdosimetry of (90)Y microspheres in man: review of four explanted whole livers. Int J Radiat Oncol Biol Phys 2004;60:1552-63. [PubMed]
- Cosimelli M, Golfieri R, Cagol PP, et al. Multi-centre phase II clinical trial of yttrium-90 resin microspheres alone in unresectable, chemotherapy refractory colorectal liver metastases. Br J Cancer 2010;103:324-31. [PubMed]
- Hendlisz A, Van den Eynde M, Peeters M, et al. Phase III trial comparing protracted intravenous fluorouracil infusion alone or with yttrium-90 resin microspheres radioembolization for liver-limited metastatic colorectal cancer refractory to standard chemotherapy. J Clin Oncol 2010;28:3687-94. [PubMed]
- van Hazel GA, Pavlakis N, Goldstein D, et al. Treatment of fluorouracil-refractory patients with liver metastases from colorectal cancer by using yttrium-90 resin microspheres plus concomitant systemic irinotecan chemotherapy. J Clin Oncol 2009;27:4089-95. [PubMed]
- Sharma RA, Van Hazel GA, Morgan B, et al. Radioembolization of liver metastases from colorectal cancer using yttrium-90 microspheres with concomitant systemic oxaliplatin, fluorouracil, and leucovorin chemotherapy. J Clin Oncol 2007;25:1099-106. [PubMed]
- Van Hazel G, Blackwell A, Anderson J, et al. Randomised phase 2 trial of SIR-Spheres plus fluorouracil/leucovorin chemotherapy versus fluorouracil/leucovorin chemotherapy alone in advanced colorectal cancer. J Surg Oncol 2004;88:78-85. [PubMed]
- Kosmider S, Tan TH, Yip D, et al. Radioembolization in combination with systemic chemotherapy as first-line therapy for liver metastases from colorectal cancer. J Vasc Interv Radiol 2011;22:780-6. [PubMed]
- Lim L, Gibbs P, Yip D, et al. A prospective evaluation of treatment with Selective Internal Radiation Therapy (SIR-spheres) in patients with unresectable liver metastases from colorectal cancer previously treated with 5-FU based chemotherapy. BMC Cancer 2005;5:132. [PubMed]
- Gulec SA, Pennington K, Wheeler J, et al. Yttrium-90 microsphere-selective internal radiation therapy with chemotherapy (chemo-SIRT) for colorectal cancer liver metastases: an in vivo double-arm-controlled phase II trial. Am J Clin Oncol 2013;36:455-60. [PubMed]
- Forner A, Ayuso C, Varela M, et al. Evaluation of tumor response after locoregional therapies in hepatocellular carcinoma: are response evaluation criteria in solid tumors reliable? Cancer 2009;115:616-23. [PubMed]
- Jaffe CC. Measures of response: RECIST, WHO, and new alternatives. J Clin Oncol 2006;24:3245-51. [PubMed]
- Gonzalez-Guindalini FD, Botelho MP, Harmath CB, et al. Assessment of liver tumor response to therapy: role of quantitative imaging. Radiographics 2013;33:1781-800. [PubMed]
- Hipps D, Ausania F, Manas DM, et al. Selective Interarterial Radiation Therapy (SIRT) in Colorectal Liver Metastases: How Do We Monitor Response? HPB Surg 2013;2013:570808.
- Kennedy AS, Coldwell D, Nutting C, et al. Resin 90Y-microsphere brachytherapy for unresectable colorectal liver metastases: modern USA experience. Int J Radiat Oncol Biol Phys 2006;65:412-25. [PubMed]
- Kennedy AS, Ball D, Steven J, et al. Safety and efficacy of resin 90Y-microspheres in 548 patients with colorectal liver metastases progressing on systemic chemotherapy. ASCO Gastrointestinal Cancers Symposium. San Francisco, California, USA, 2013:Abs 264.
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205-16. [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [PubMed]
- Coldwell DM, Sewell PE. The expanding role of interventional radiology in the supportive care of the oncology patient: from diagnosis to therapy. Semin Oncol 2005;32:169-73. [PubMed]
- Kennedy A, Nag S, Salem R, et al. Recommendations for radioembolization of hepatic malignancies using yttrium-90 microsphere brachytherapy: a consensus panel report from the radioembolization brachytherapy oncology consortium. Int J Radiat Oncol Biol Phys 2007;68:13-23. [PubMed]
- Salem R, Thurston KG, Carr BI, et al. Yttrium-90 microspheres: radiation therapy for unresectable liver cancer. J Vasc Interv Radiol 2002;13:S223-9. [PubMed]
- Gray B, Van Hazel G, Hope M, et al. Randomised trial of SIR-Spheres plus chemotherapy vs. chemotherapy alone for treating patients with liver metastases from primary large bowel cancer. Ann Oncol 2001;12:1711-20. [PubMed]
- Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457-81.
- Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:303-12. [PubMed]
- Miller FH, Keppke AL, Reddy D, et al. Response of liver metastases after treatment with yttrium-90 microspheres: role of size, necrosis, and PET. AJR Am J Roentgenol 2007;188:776-83. [PubMed]
- Atassi B, Bangash AK, Bahrani A, et al. Multimodality imaging following 90Y radioembolization: a comprehensive review and pictorial essay. Radiographics 2008;28:81-99. [PubMed]
- Guo Y, Yaghmai V, Salem R, et al. Imaging tumor response following liver-directed intra-arterial therapy. Abdom Imaging 2013;38:1286-99. [PubMed]
- Kalva SP, Rana RS, Liu R, et al. Yttrium-90 Radioembolization as Salvage Therapy for Liver Metastases From Colorectal Cancer. Am J Clin Oncol 2014. [Epub ahead of print]. [PubMed]
- Saxena A, Meteling B, Kapoor J, et al. Is yttrium-90 radioembolization a viable treatment option for unresectable, chemorefractory colorectal cancer liver metastases? A large single-center experience of 302 patients. Ann Surg Oncol 2015;22:794-802. [PubMed]
- Sofocleous CT, Garcia AR, Pandit-Taskar N, et al. Phase I trial of selective internal radiation therapy for chemorefractory colorectal cancer liver metastases progressing after hepatic arterial pump and systemic chemotherapy. Clin Colorectal Cancer 2014;13:27-36. [PubMed]
- Benson AB 3rd, Geschwind JF, Mulcahy MF, et al. Radioembolisation for liver metastases: results from a prospective 151 patient multi-institutional phase II study. Eur J Cancer 2013;49:3122-30. [PubMed]
- Smits ML, van den Hoven AF, Rosenbaum CE, et al. Clinical and laboratory toxicity after intra-arterial radioembolization with (90)y-microspheres for unresectable liver metastases. PLoS One 2013;8:e69448. [PubMed]
- Seidensticker R, Denecke T, Kraus P, et al. Matched-pair comparison of radioembolization plus best supportive care versus best supportive care alone for chemotherapy refractory liver-dominant colorectal metastases. Cardiovasc Intervent Radiol 2012;35:1066-73. [PubMed]
- Martin LK, Cucci A, Wei L, et al. Yttrium-90 radioembolization as salvage therapy for colorectal cancer with liver metastases. Clin Colorectal Cancer 2012;11:195-9. [PubMed]
- Zerizer I, Al-Nahhas A, Towey D, et al. The role of early 18F-FDG PET/CT in prediction of progression-free survival after 90Y radioembolization: comparison with RECIST and tumour density criteria. Eur J Nucl Med Mol Imaging 2012;39:1391-9. [PubMed]
- Nace GW, Steel JL, Amesur N, et al. Yttrium-90 radioembolization for colorectal cancer liver metastases: a single institution experience. Int J Surg Oncol 2011;2011:571261.
- Cianni R, Urigo C, Notarianni E, et al. Selective internal radiation therapy with SIR-spheres for the treatment of unresectable colorectal hepatic metastases. Cardiovasc Intervent Radiol 2009;32:1179-86. [PubMed]
- Jakobs TF, Hoffmann RT, Dehm K, et al. Hepatic yttrium-90 radioembolization of chemotherapy-refractory colorectal cancer liver metastases. J Vasc Interv Radiol 2008;19:1187-95. [PubMed]
- Chua TC, Bester L, Saxena A, et al. Radioembolization and systemic chemotherapy improves response and survival for unresectable colorectal liver metastases. J Cancer Res Clin Oncol 2011;137:865-73. [PubMed]
- Mulcahy MF, Lewandowski RJ, Ibrahim SM, et al. Radioembolization of colorectal hepatic metastases using yttrium-90 microspheres. Cancer 2009;115:1849-58. [PubMed]
- Stubbs RS, O'Brien I, Correia MM. Selective internal radiation therapy with 90Y microspheres for colorectal liver metastases: single-centre experience with 100 patients. ANZ J Surg 2006;76:696-703. [PubMed]
- Lewandowski RJ, Thurston KG, Goin JE, et al. 90Y microsphere (TheraSphere) treatment for unresectable colorectal cancer metastases of the liver: response to treatment at targeted doses of 135-150 Gy as measured by [18F]fluorodeoxyglucose positron emission tomography and computed tomographic imaging. J Vasc Interv Radiol 2005;16:1641-51. [PubMed]
- Van Cutsem E, Rivera F, Berry S, et al. Safety and efficacy of first-line bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in metastatic colorectal cancer: the BEAT study. Ann Oncol 2009;20:1842-7. [PubMed]
- Chalian H, Töre HG, Horowitz JM, et al. Radiologic assessment of response to therapy: comparison of RECIST Versions 1.1 and 1.0. Radiographics 2011;31:2093-105. [PubMed]
- Duke E, Deng J, Ibrahim SM, et al. Agreement between competing imaging measures of response of hepatocellular carcinoma to yttrium-90 radioembolization. J Vasc Interv Radiol 2010;21:515-21. [PubMed]
- Kamel IR, Reyes DK, Liapi E, et al. Functional MR imaging assessment of tumor response after 90Y microsphere treatment in patients with unresectable hepatocellular carcinoma. J Vasc Interv Radiol 2007;18:49-56. [PubMed]
- Rhee TK, Naik NK, Deng J, et al. Tumor response after yttrium-90 radioembolization for hepatocellular carcinoma: comparison of diffusion-weighted functional MR imaging with anatomic MR imaging. J Vasc Interv Radiol 2008;19:1180-6. [PubMed]
- Dudeck O, Zeile M, Wybranski C, et al. Early prediction of anticancer effects with diffusion-weighted MR imaging in patients with colorectal liver metastases following selective internal radiotherapy. Eur Radiol 2010;20:2699-706. [PubMed]
- Wybranski C, Zeile M, Löwenthal D, et al. Value of diffusion weighted MR imaging as an early surrogate parameter for evaluation of tumor response to high-dose-rate brachytherapy of colorectal liver metastases. Radiat Oncol 2011;6:43. [PubMed]
- Löwenthal D, Zeile M, Lim WY, et al. Detection and characterisation of focal liver lesions in colorectal carcinoma patients: comparison of diffusion-weighted and Gd-EOB-DTPA enhanced MR imaging. Eur Radiol 2011;21:832-40. [PubMed]
- Tajima T, Akahane M, Takao H, et al. Detection of liver metastasis: is diffusion-weighted imaging needed in Gd-EOB-DTPA-enhanced MR imaging for evaluation of colorectal liver metastases? Jpn J Radiol 2012;30:648-58. [PubMed]
- Szyszko T, Al-Nahhas A, Canelo R, et al. Assessment of response to treatment of unresectable liver tumours with 90Y microspheres: value of FDG PET versus computed tomography. Nucl Med Commun 2007;28:15-20. [PubMed]
- Tochetto SM, Töre HG, Chalian H, et al. Colorectal liver metastasis after 90Y radioembolization therapy: pilot study of change in MDCT attenuation as a surrogate marker for future FDG PET response. AJR Am J Roentgenol 2012;198:1093-9. [PubMed]
- Dezarn WA, Cessna JT, DeWerd LA, et al. Recommendations of the American Association of Physicists in Medicine on dosimetry, imaging, and quality assurance procedures for 90Y microsphere brachytherapy in the treatment of hepatic malignancies. Med Phys 2011;38:4824-45. [PubMed]
- Fendler WP, Philippe Tiega DB, Ilhan H, et al. Validation of several SUV-based parameters derived from 18F-FDG PET for prediction of survival after SIRT of hepatic metastases from colorectal cancer. J Nucl Med 2013;54:1202-8. [PubMed]
- Morsbach F, Pfammatter T, Reiner CS, et al. Computed tomographic perfusion imaging for the prediction of response and survival to transarterial radioembolization of liver metastases. Invest Radiol 2013;48:787-94. [PubMed]