
Rectal cancer in young patients: incidence and outcome disparities
Introduction
Excluding skin cancer, colorectal cancer (CRC) is the third most frequent cancer in the United States, with an estimated incidence of 140,250 patients in 2018, with an estimated 43,030 cases being rectal cancers (1). Moreover, CRC is the third most common cause of mortality among both sexes. However, the incidence of CRC has been steadily decreasing, largely related to colorectal screening. Gilbertsen et al. reported in 1978 that annual proctosigmoidoscopic examination and polyp removal prevented 95% of anticipated rectal cancer and, of the cancers that were identified, 80% were minimally invasive with submucosal involvement only (2). Subsequently, the National Polyp Study Workgroup published a reduction in incidence of CRC with colonoscopic polypectomy (3). Consequently, with implementation of screening colonoscopy in 1997, rectal cancer incidence has decreased overall. However, in recent studies, it has been shown that the incidence of CRC has increased among patients younger than 50 and decreasing among older patients (4). Per the National Cancer Institute (NCI), the number of young-onset (<50 years old) CRC cases have increased by about 51% since 1994 (5) Patients born around 1990 have quadruple the risk of rectal cancer compared to those born in 1950, with an incidence rate ratio of 4.3 (95% CI: 2.2–8.5) (4).
Furthermore, younger patients diagnosed with CRC tend to present with hematochezia, obstruction, and abdominal discomfort (6), and may have a 1.4 fold delay in time to diagnosis, compared to older patients (7). Additionally, younger patients with colon cancer tend to have more aggressive pathological features, including: lymphovascular invasion, T3/T4 tumors, lymph node metastases and stage III disease (8), hence they are more often diagnosed with advanced disease (9).
Until recently, CRC screening was recommended to start at the age of 50 (10,11). However, given the concerning rise in incidence of CRC among young adults, the American Cancer Society (ACS) recently updated their recommendations to start screening patients at 45 years old for individuals with average risk (12). The purpose of this study is to further investigate disparate incidence trends among young rectal cancer patients using the Surveillance, Epidemiology, and End Results Analysis (SEER) database and then evaluate survival outcomes using both the SEER and National Cancer Database (NCDB) databases. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jgo-20-197).
Methods
Data source
The NCDB is a database that records cancer data from >1,500 Commission on Cancer (COC)-accredited facilities nationwide. The database encompasses >70% of newly diagnosed cancer cases and reports a number of clinical parameters, including: demographics, staging, course of treatment and overall survival (OS).
In contrast, the SEER Program collects and publishes cancer incidence and survival data from population-based cancer registries covering approximately 34% of the US population. The SEER 9 (1975 to 2016) and SEER 18 (2000 to 2016) registries were used for incidence and annual percent change (APC) calculations. The specialized Radiation/Chemotherapy Database (SEER 18 Custom Data, November 2017 Submission) was used for clinical outcomes analysis (13).
Cohort analyzed
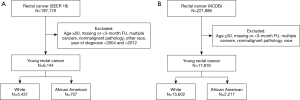
The selected cohort consisted of young (<50), white or African American patients with International Classification of Disease for Oncology, 3rd Edition (ICD-O-3/WHO 2008) diagnosis of ‘Rectum’ cancer. Patients with missing or <3 months of follow-up, multiple cancers and nonmalignant pathology were excluded (Figure 1).
Incidence analysis
Age-adjustment was performed using the 2000 U.S. Standard Population. APC was calculated and heteroscedasticity accounted for using weighted least squares regression (14). Modified gamma and F intervals for confidence interval estimation was performed using the Tiwari modification (15) in SEER*Stat [Surveillance Research Program, National Cancer Institute SEER*Stat software (seer.cancer.gov/seerstat) version 8.3.5]. In order to better fit the APC trends over time, Joinpoint regression modeling was performed with log-linear transformation and final model selection via Monte Carlo Permutation method (16) (Joinpoint Regression Program, Version 4.6.0.0. April, 2018; Statistical Research and Applications Branch, National Cancer Institute). If the number of incident cases were low, a 0 joinpoint curve was selected. Percent changes were calculated using 1-year for each endpoint.
Outcomes analysis
Patient characteristics were evaluated before and after matching by using a combination of Chi square analysis and standard mean difference (SMD), with a SMD >0.1 determined to be imbalanced (17). Univariate analysis (UVA) of clinical parameters effect on OS was performed using the Kaplan-Meier (KM) method, with the log rank method to assess for significance (18). Statistical significance was accepted at P<0.05 and 2-sided tests were used for all analyses. The following clinical parameters were evaluated: age, facility type, insurance, income, percent with no high school diploma, population density, Charlson/Deyo Comorbid Conditions (NCDB only), marital status (SEER only), percent under poverty level (SEER only) sex, year of diagnosis, grade, stage, circumferential margin status, chemotherapy, radiation therapy, and surgery.
Multivariable analysis (MVA) of clinical parameters and OS was performed using Cox proportional hazards regression modeling. For SEER 18 patients, cancer-specific survival (CSS) was also determined. Covariates included in the final MVA model were selected via backward elimination, excluding covariates with P>0.1.
In order to reduce indication bias, binary logistic regression was used to calculate propensity scores (PS) for being white or African American (19). Subsequently, inverse probability of treatment weights (IPTW) were calculated as 1/PS and 1/(1-PS) (20). Finally, IPTW-adjusted UVA KM and doubly robust MVA cox proportional hazards regression modeling was performed (21-23). Subgroup analyses were assessed for heterogeneity using I2. Cases with incomplete/missing data were excluded.
Statistical analysis
All statistics were completed using SEER*Stat (v8.3.5, The Surveillance Research Program of the Division of Cancer Control and Population Sciences, National Cancer Institute), Joinpoint Regression Program (v4.7.0.0, Statistical Research and Applications Branch, National Cancer Institute), SPSS (v24, IBM), RStudio (v1.2.1335). The following R packages were used: survminer, survival, ggplot2, tableone, ipw, IPWsurvival, and olsrr. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The SEER and NCDB registries provide de-identified data. Consequently, this study does not require Institutional Review Board (IRB) review or approval. R markdown and data for all analyses are available upon request.
Results
Patient characteristics
A total of 6,144 young (<50 years old), white or African American patients from 2004 to 2012 were identified from the SEER 18 registry with an ICD-O-3 site code of C209, corresponding to rectal cancer (Figure 1A) and a total of 17,819 young white or African American patients from 2004 to 2012 were identified from the NCDB (Figure 1B). The median follow-up for the NCDB and SEER cohorts were 44 months (range, 3–130.5 months) for and 58 months (range, 3–143 months), respectively. Patient characteristics for both cohorts are shown in Table 1 (unadjusted and adjusted characteristics or the NCDB and SEER cohorts, stratified by race, can be found in Tables S1,S2, respectively). The median age at diagnosis was 44 for both cohorts, and the majority of patients were white in the NCDB (87.6%) and SEER (88.5%) cohorts. There was a male predominance in both NCDB and SEER, 56.9% and 58.1%, respectively. Similarly, most patients were diagnosed with Stage III rectal cancer in the NCDB (32.6%) and SEER (35.2%) cohorts.

Full table
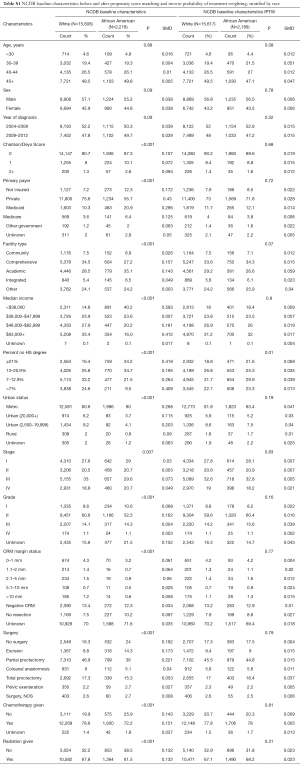
Full table
Full table
Rectal cancer incidence
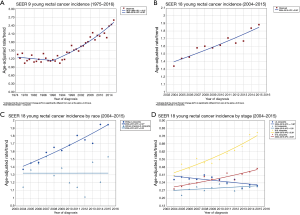
Analysis of the SEER 9 registries revealed that the incidence of rectal cancer among young patients did not change significantly from 1975 to 1990, with an age-adjusted APC of −0.67 (P=0.3). However, from 1990 to 2016, there was a significant change in rectal cancer incidence, with a steadily increasing APC of 3.06 (Figure 2A, P<0.05). Similarly, analysis of the SEER 18 registries confirmed a significant increase from 2004 to 2015 in rectal cancer incidence among young patients with an APC of 2.58 (Figure 2B, P<0.05). Interestingly, there was no overall change in age-adjusted APC among young African American patients (APC 0.00, P=1); however, there was a significant increase among young white patients (Figure 2C, APC 2.97, P<0.05). As depicted in Figure 2D, there was an overall reduction in stage I disease (APC −1.96, P<0.05) and stable incidence of stage II disease (APC 1.26, P=0.1). Additionally, there was an overall increase for both stage III and IV among young rectal cancer patients, with an age-adjusted APC of 5.35 and 3.83, respectively (P<0.05). After stratifying by race there remained an increase in age-adjusted incidence of stage III (APC 5.57, P<0.05) and IV (APC 4.66, P<0.05) rectal cancer among young white patients (Figure S1A) and also an overall increase in age-adjusted incidence of stage III cancer for 2010 to 2015 (APC 14.57, P<0.05) among young African American patients (Figure S1B).

Cohort characteristics and univariate analysis
The baseline characteristics among young white and African American patients in the NCDB and SEER cohorts are depicted in Tables S2,S3, respectively. After PS matching and IPTW-adjustment, baseline factors were comparable between both databases.
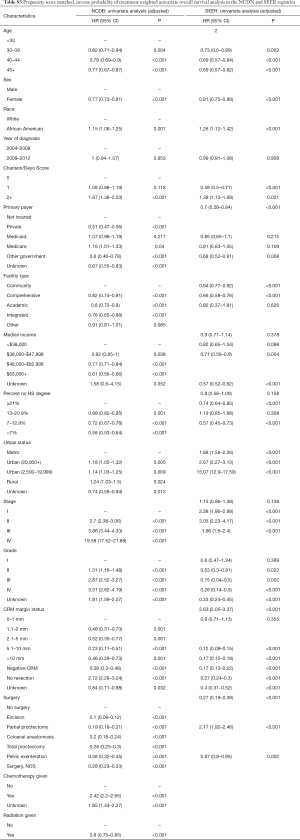
Full table
The impact of patient, tumor and treatment characteristic on OS were assessed using IPTW-adjusted KM (Table S3). Prior to PS-matching and IPTW-adjustment, the following covariates significantly impacted OS in the NCDB cohort: age, sex, race, CDCC, insurance status, facility type, median income, percent no HS degree, urban location, stage, grade, circumferential margin (CRM) status, type of surgery, chemotherapy and radiation therapy. Within the SEER cohort: age, sex, race, insurance, income, percent no HS degree, marital status, stage, grade, CRM status, type of surgery, chemotherapy and radiation therapy had an impact on OS.
Multivariable analysis
A complete summary of the doubly robust IPTW-adjusted MVA analysis is shown in Table 2. Factors that were strongly protective in the NCDB cohort include: female sex (HR 0.82, 95% CI: 0.78–0.87, P<0.001), private insurance (HR 0.73, 95% CI: 0.66–0.8, P<0.001), negative CRM (HR 0.54, 95% CI: 0.44–0.66, P<0.001), any surgery, and radiation therapy (HR 0.87, 95% CI: 0.81–0.94, P<0.001). Factors strongly associated with worse OS in the NCDB cohort: African American race (HR 1.17, 95% CI: 1.1–1.3, P<0.001), CDCC 2+ (HR 1.5, 95% CI: 1.2–1.8, P<0.001), stage III (HR 3.6, 95% CI: 3.2–4.1, P<0.001) and stage IV (HR 12.7, 95% CI: 11.2–14.5, P<0.001).
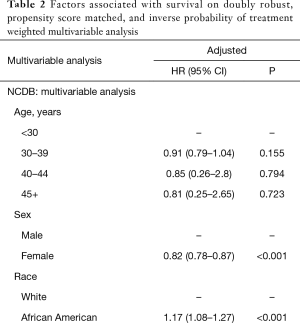
Full table
Covariates that were strongly protective in the SEER cohort include: female sex (HR 0.87, 95% CI: 0.8–0.95, P<0.001), married status (HR 0.72, 95% CI: 0.65–0.79, P<0.001), negative CRM (HR 0.44, 95% CI: 0.32–0.6, P<0.001). Moreover, factors strongly associated with worse OS include: African American race (HR 1.4, 95% CI: 1.2–1.6, P<0.001), stage III (HR 2.7, 95% CI: 2.2–3.2, P<0.001) and stage IV (HR 9.6, 95% CI: 8–11.5, P<0.001).
Overall survival
After propensity score matching, the 5- and 10-year OS for the entire NCDB cohort was 69.4% and 56.4%, respectively. For the entire SEER cohort, the 5- and 10-year OS was 65.6% and 56.3%, respectively. Young African American rectal cancer patients had worse overall survival in both the NCDB (HR 1.1, 95% CI: 1.1–1.2, P=0.01) and SEER (HR 1.3, 95% CI: 1.1–1.4, P=0.002) databases. However, after stratifying by stage at diagnosis, only stage III patients were found to have an overall survival disparity between whites and African Americans (Figure 3). Within the NCDB stage III cohort, the median overall survival for whites was not reached compared to 120 months among African American patients (HR 1.4, 95% CI: 1.3–1.7, P<0.001). Similarly, within the SEER stage III cohort, the median overall survival was not reached compared to 96 months among African American patients (HR 1.6, 95% CI: 1.3–2, P<0.001).
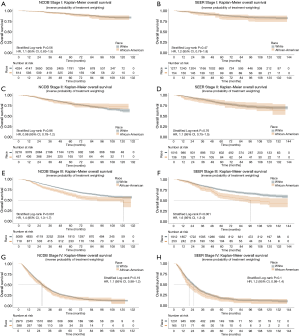
Cancer-specific survival
For the SEER cohort, after propensity score matching, the 5- and 10-year CSS was 66.2% and 56.8% respectively. White race had improved CSS compared to African Americans before (HR 1.5, 95% CI: 1.4–1.7, P<0.001) and after propensity score matching (HR 1.2, 95% CI: 1.1–1.4, P=0.005). However, when stratified by stage at diagnosis, only stage III patients demonstrated a difference in CSS (HR 1.5, 95% CI: 1.2–1.9, P=0.002). The estimated 10-year CSS for stage III rectal cancer among whites compared to African Americans was 57.7% vs. 49.9% (P=0.002) respectively. There were no statistically significant differences between whites and African Americans in CSS among stage I, II, or IV patients (Figure S2).
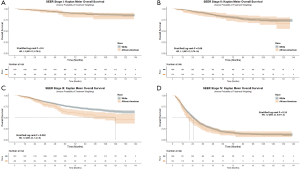
Discussion
With over 20,000 young rectal cancer patients assessed, this is one of the largest, contemporary cohort analyses evaluating trends in incidence and outcome disparities over time. While the overall incidence of rectal cancer among young patients is rising, our analysis demonstrates that this is driven predominantly by young white patients, with an APC of approximately 3%. In addition, there appears to be a significant increase in stage III and IV rectal cancer among young white patients with APC of 5.4% and 3.8%, respectively, while there is significant increase in stage III rectal cancer among African Americans. Despite the disparity of increasing incidence of rectal cancer among young white patients, young African American patients have worse outcomes in both the NCDB and SEER databases. Moreover, we implemented robust statistical techniques with PS-matching and IPTW-adjustment to mitigate indication bias between white and African American patients. This revealed that young stage III rectal cancer patients have disparate outcomes in terms of OS for both NCDB/SEER cohorts as well as CSS for the SEER cohort. This combined data analysis further contributes to the growing body of literature that identifies increasing incidence of rectal cancer among young patients.
In addition to race and stage, there were a number of other patient and treatment characteristics that portended worse overall survival, many of which are possibly surrogates for overall socioeconomic status and performance status. Specifically, Medicare (which for the cohort analyzed would only be available to patients receiving social security disability insurance or who have end-stage renal disease or amyotrophic lateral sclerosis), CDCC index, and median income <$48,000 were all associated with worse OS, even on IPTW-adjusted MVA. Moreover, patients that did not receive surgery and those with close/positive CRM margins (0–1 mm) had worse outcomes.
Recently, Virostko et al. published an NCDB analysis of trends in age of CRC from 2004–2015 (24). They noted younger patients were more likely to have stage III/IV disease compared to older patients (52% vs. 40%). Notably, this study is unable to capture true epidemiologic metrics as the NCDB does not collect population data. Crosbie et al. evaluated SEER and New Jersey State Cancer Registry and identified an increase in rectal cancer incidence among young patients in both cohorts (25). While both of these studies provide important insight into the trends and incidence of CRC among young patients, neither evaluated outcome disparities.
African Americans are reported to have increased incidence of CRC and worse OS compared to whites (26). Indeed, Rahman et al. reported on increased CRC incidence among young minorities and worse OS among African Americans compared to whites (5-year OS 56% vs. 66%, P<0.0001) (27). However, this study reported on raw survival outcomes and provided point-estimates at 1- and 5-year. Holowatyj published a SEER analysis comparing racial/ethnic survival disparities among young patients with CRC (28). They also identified an improvement in both OS and CSS among young white compared to African American patients. In contrast, Kolarich et al. analyzed the NCDB (2004 to 2014) and noted that African American young rectal cancer patients had worse OS on univariate analysis, but not on multivariable analysis (HR 1.1, 95% CI: 0.8–1.5) (29). Nevertheless, covariate adjustment via Cox proportional hazards modeling yields HRs that are estimates of a conditional effect, and do not necessarily reflect marginal effects at the population level (30).
In contrast to the above SEER and NCDB analyses, the strength of the current study is the combined analysis of both databases and the ability to estimate marginal HR via propensity score matching and inverse probability of treatment weighting. Importantly, the incidence trends in the SEER 9 and SEER 18 registries were validated in the non-population based NCDB cohort. Furthermore, after adjusting for confounding factors, all baseline characteristics were similar among both cohorts, thus allowing for adjusted univariate and doubly robust multivariable analyses to further understand the impact of race on OS and CSS. These robust statistical analyses identified young African American patients to have worse OS (doubly robust HR 1.4, 95% CI: 1.2–-1.6, P<0.001) compared to young white patients. These findings were further validated using the NCDB database, in which the doubly robust HR was 1.2 (95% CI: 1.1–1.3), P<0.001. Another strength of the current study is a focused analysis on rectal cancer as these tumors have very different treatment approaches and outcomes.
There are multiple hypotheses that attempt to explain the underlying force responsible for the striking increase in rectal cancer incidence among young patients. Recently, Liu et al. reported on the association of obesity and early-onset CRC using The Nurses Health Study II. They found that for every 5-point increase in BMI, there was an associated 20% increase in CRC (31). Notably, obesity in the US has increased significantly from 1980 to 2000 and 2003 to 2004 (32). Indeed, a recent report of the National Health and Nutrition Examination Survey identified age-adjusted prevalence of obesity of 36.5% and 40.8% for men and women, respectively (33). Unfortunately, neither the NCDB or SEER databases record information on height, weight, or BMI. Metabolic syndrome and insulin resistance, both of which are highly correlated with obesity, also seem to be associated with an increase in CRC incidence (34). Likewise, poor nutrition (35) and a sedentary lifestyle (36) have also been associated with increased incidence of CRC. Recently, Willauer et al. showed that the molecular background of tumors are different in adults vs. young patients (37). Moreover, tumors were molecularly distinct among subsets of young adults.
SEER and NCDB data have a tremendous quality control mechanism. Nevertheless, the data are dependent on precise coding as well as reporting, and are at risk for reporting bias. Furthermore, despite attempts to control for confounding variables, there are likely multiple unmeasured and unknown confounders that cannot be adequately controlled for. For instance, family history of cancer, BMI, diet, genetics and comorbid medical conditions are not recorded and, therefore, cannot be used to adjust for confounding. As reported, age-adjusted obesity is more prevalent among African Americans compared to whites for all ages (48% vs. 37%), 20–39 years old (43% vs. 32%), 40–59 years old (54% vs. 41%), and 60+ years old (47% vs. 40%) (33). Nevertheless, this study is one of the largest studies to date, and to our knowledge, the only combined SEER/NCDB cohort analysis demonstrating consistent outcomes in both databases.
Conclusions
The current study adds to the growing body of literature demonstrating an alarming increase in incidence of rectal cancer among young patients. Moreover, the incidence appears to be increasing particularly among young white patients and driven by stage III disease. After controlling for confounding variables, we identified outcome disparities among young African American patients with stage III rectal cancer, compared to matched white patients. The etiology of this disparity remains to be characterized but may relate to observed trends in nutrition and obesity however other risk factors can play a role. Further research into the link between obesity and rectal cancer is greatly needed and may further inform a risk-adapted screening program.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors present the study in accordance with the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jgo-20-197
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jgo-20-197). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The SEER/NCDB registries provide de-identified data. Consequently, this study does not require Institutional Review Board (IRB) review or approval.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Gilbertsen VA, Nelms JM. The prevention of invasive cancer of the rectum. Cancer 1978;41:1137-9. [Crossref] [PubMed]
- Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med 1993;329:1977-81. [Crossref] [PubMed]
- Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal Cancer Incidence Patterns in the United States, 1974-2013. J Natl Cancer Inst 2017;109:djw322. [Crossref] [PubMed]
- Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin 2017;67:177-93. [Crossref] [PubMed]
- Myers EA, Feingold DL, Forde KA, et al. Colorectal cancer in patients under 50 years of age: A retrospective analysis of two institutions' experience. World J Gastroenterol 2013;19:5651-7. [Crossref] [PubMed]
- Chen FW, Sundaram V, Chew TA, et al. Advanced-Stage Colorectal Cancer in Persons Younger Than 50 Years Not Associated With Longer Duration of Symptoms or Time to Diagnosis. Clin Gastroenterol Hepatol 2017;15:728-37.e3. [Crossref] [PubMed]
- Rodriguez L, Brennan K, Karim S, et al. Disease Characteristics, Clinical Management, and Outcomes of Young Patients With Colon Cancer: A Population-based Study. Clin Colorectal Cancer 2018;17:e651-61. [Crossref] [PubMed]
- Fu J, Yang J, Tan Y, et al. Young patients (≤ 35 years old) with colorectal cancer have worse outcomes due to more advanced disease: a 30-year retrospective review. Medicine (Baltimore) 2014;93:e135. [Crossref] [PubMed]
- Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2016;315:2564-75. [Crossref] [PubMed]
- Rex DK, Boland CR, Dominitz JA, et al. Colorectal Cancer Screening: Recommendations for Physicians and Patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol 2017;112:1016-30. [Crossref] [PubMed]
- Wolf AMD, Fontham ETH, Church TR, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin 2018;68:250-81. [Crossref] [PubMed]
- Quinn TJ, Almahariq MF, Siddiqui ZA, et al. Trimodality therapy for atypical teratoid/rhabdoid tumor is associated with improved overall survival: A surveillance, epidemiology, and end results analysis. Pediatr Blood Cancer 2019;66:e27969. [Crossref] [PubMed]
- Rao CR, Toutenburg H, Heumann C. Linear Models and Generalizations: Least Squares and Alternatives. 3rd ed. Berlin Heidelberg: Springer-Verlag, 2008.
- Tiwari RC, Clegg LX, Zou Z. Efficient interval estimation for age-adjusted cancer rates. Stat Methods Med Res 2006;15:547-69. [Crossref] [PubMed]
- Kim HJ, Fay MP, Feuer EJ, et al. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med 2000;19:335-51. [Crossref] [PubMed]
- Austin PC. Goodness-of-fit diagnostics for the propensity score model when estimating treatment effects using covariate adjustment with the propensity score. Pharmacoepidemiol Drug Saf 2008;17:1202-17. [Crossref] [PubMed]
- Efron B. Logistic Regression, Survival Analysis, and the Kaplan-Meier Curve. J Am Stat Assoc 1988;83:414-25. [Crossref]
- Rosenbaum PR. Propensity Score. Encyclopedia of Biostatistics: American Cancer Society, 2005.
- Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol 2008;168:656-64. [Crossref] [PubMed]
- Almahariq MF, Quinn TJ, Siddiqui Z, et al. Breast conserving therapy is associated with improved overall survival compared to mastectomy in early-stage, lymph node-negative breast cancer. Radiother Oncol 2020;142:186-94. [Crossref] [PubMed]
- Almahariq MF, Quinn TJ, Siddiqui ZA, et al. Post-mastectomy radiotherapy is associated with improved overall survival in T3N0 patients who do not receive chemotherapy. Radiother Oncol 2020;145:229-37. [Crossref] [PubMed]
- Quinn TJ, Rajagopalan MS, Gill B, et al. Patterns of care and outcomes for adjuvant treatment of pT3N0 rectal cancer using the National Cancer Database. J Gastrointest Oncol 2020;11:1-12. [Crossref] [PubMed]
- Virostko J, Capasso A, Yankeelov TE, et al. Recent trends in the age at diagnosis of colorectal cancer in the US National Cancer Data Base, 2004-2015. Cancer 2019;125:3828-35. [Crossref] [PubMed]
- Crosbie AB, Roche LM, Johnson LM, et al. Trends in colorectal cancer incidence among younger adults-Disparities by age, sex, race, ethnicity, and subsite. Cancer Med 2018;7:4077-86. [Crossref] [PubMed]
- Agrawal S, Bhupinderjit A, Bhutani MS, et al. Colorectal cancer in African Americans. Am J Gastroenterol 2005;100:515-23; discussion 514. [Crossref] [PubMed]
- Rahman R, Schmaltz C, Jackson CS, et al. Increased risk for colorectal cancer under age 50 in racial and ethnic minorities living in the United States. Cancer Med 2015;4:1863-70. [Crossref] [PubMed]
- Holowatyj AN, Ruterbusch JJ, Rozek LS, et al. Racial/Ethnic Disparities in Survival Among Patients With Young-Onset Colorectal Cancer. J Clin Oncol 2016;34:2148-56. [Crossref] [PubMed]
- Kolarich A, George TJ, Hughes SJ, et al. Rectal cancer patients younger than 50 years lack a survival benefit from NCCN guideline-directed treatment for stage II and III disease. Cancer 2018;124:3510-9. [Crossref] [PubMed]
- Austin PC. The performance of different propensity score methods for estimating marginal hazard ratios. Stat Med 2013;32:2837-49. [Crossref] [PubMed]
- Liu PH, Wu K, Ng K, et al. Association of Obesity With Risk of Early-Onset Colorectal Cancer Among Women. JAMA Oncol 2019;5:37-44. [Crossref] [PubMed]
- Flegal KM, Kruszon-Moran D, Carroll MD, et al. Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA 2016;315:2284-91. [Crossref] [PubMed]
- Hales CM, Fryar CD, Carroll MD, et al. Differences in Obesity Prevalence by Demographic Characteristics and Urbanization Level Among Adults in the United States, 2013-2016. JAMA 2018;319:2419-29. [Crossref] [PubMed]
- Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. Diabetes Care 2010;33:1674-85. [Crossref] [PubMed]
- Vargas AJ, Thompson PA. Diet and nutrient factors in colorectal cancer risk. Nutr Clin Pract 2012;27:613-23. [Crossref] [PubMed]
- Lynch BM. Sedentary behavior and cancer: a systematic review of the literature and proposed biological mechanisms. Cancer Epidemiol Biomarkers Prev 2010;19:2691-709. [Crossref] [PubMed]
- Willauer AN, Liu Y, Pereira AAL, et al. Clinical and molecular characterization of early-onset colorectal cancer. Cancer 2019;125:2002-10. [Crossref] [PubMed]


