Extra Gastrointestinal Stromal Tumor treated with imatinib in a patient with Neurofibromatosis type 1
Introduction
Neurofibromatosis type 1 (NF1), also known as Von- Recklinghausen’s disease is one of the most commonly transmitted hereditary autosomal dominant diseases, with an estimated birth incidence of 1:3,000. The pathogenesis is thought to be due to a mutation in the NF1 tumor suppressor gene that is found on chromosome 17. This mutation leads to the loss of tumor suppressor function, which then results in the development of benign and malignant tumors. In addition to cutaneous, soft tissue, and visceral (plexiform) neurofibromas, this syndrome is connected with several types of gastrointestinal (GI) and abdominal tumors. Examples of such include the following: neuronal hyperplasia (neuromas), ampullary carcinoid, pheochromocytoma, and gastrointestinal stromal tumor (GIST). The latter has been suggested to be the most common NF1-associated GI tumor. Here we present the case of a NF1 patient who was found to have extra gastrointestinal stromal tumor (EGIST) which is seen in <5% cases of GIST.
Case report
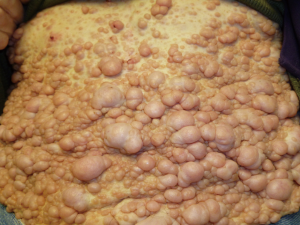
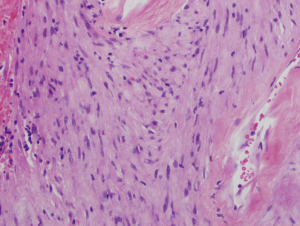
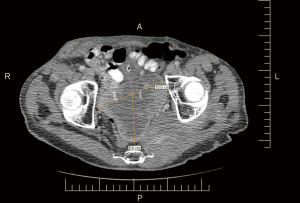
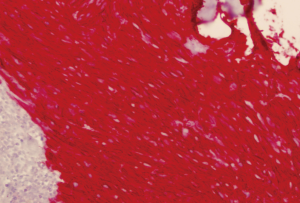
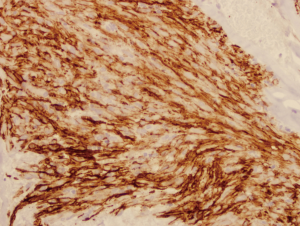
A 64-year-old man with known Neurofibromatosis type 1 was brought to the hospital after he was found unconscious and pulseless. He had multiple cutaneous neurofibromas (Figure 1). He was revived with CPR and defibrillation. He then underwent cardiac catheterization which revealed three-vessel coronary artery disease and was recommended to undergo coronary artery bypass graft (CABG) surgery. During the course of acute management, CT scans of the thorax and the abdomen and pelvis were obtained to rule out any hemorrhage or aortic dissection. Note was made of a large inhomogeneous pelvic mass with dimensions of 8.6 cm × 10 cm × 7.8 cm (Figure 2). A CT-guided biopsy of the mass revealed palisaded-appearing long spindle cells (Figure 3). A schwannoma was considered on morphologic grounds, but an S-100 stain was negative. There was focal, weak staining for smooth muscle actin (SMA). The neoplastic cells were strongly and diffusely positive for CD117 (c-KIT) (Figure 4) and CD34 (Figure 5), indicating a GIST. The KIT and PDGFR mutations were found to be negative on the mutational analysis. The tumor was considered to be marginally resectable and so the patient was started on imatinib 400 mg daily with the hope of making subsequent surgery feasible. A repeat CT abdomen/pelvis done after 3 months of imatinib therapy, showed multiple foci of air suggestive of necrosis, though the size of the tumor remained stable. The tumor was then resected en-bloc. A cavity was noted within the tumor along with fistula formation necessitating excision of part of the small intestine. After the surgery he was restarted on imatinib 400 mg daily with surveillance CT scans planned every six months.
Discussion
Gastrointestinal stromal tumors (GIST) are mesenchymal neoplasms that are related to the interstitial cells of Cajal of the myenteric plexus. These tumors express the cell-surface transmembrane receptor c-KIT that has tyrosine kinase activity and is the protein product of the KIT proto-oncogene (1).
GIST are rare tumors with an incidence of 1.5/100,000/year with EGIST being <5% of the total. There is a well-known correlation between NF1 and GIST as GIST develops in 7% of patients with NF1. The occurrence of NF1 is 150-180 times more frequent in GIST than in the general population. However, it is known that NF1- associated and sporadic GIST have different pathogenesis.
EGIST are very rare mesenchymal tumors which originate in sites outside the gastrointestinal tract, with clinico-pathological and molecular profiles similar to GIST. The most common sites of EGIST are the retroperitoneum, the mesentery and the omentum (2). However, other less frequent sites have also been reported such as the gallbladder, the pancreas and the recto-vaginal septum. The EGIST comprise a group of aggressive stromal tumors; their behavior is similar to those of GIST of distal location. It is unusual to diagnose EGIST when they are small due to their atypical location and vague symptomatology (2). Goh et al. in a series of 8 cases found average tumor size of 14.8 cm at the time of diagnosis (3).
NF1-associated GIST appears to be a different entity than sporadic GIST (4). NF1 patients develop GIST at a younger age (median, 49 years) than individuals with sporadic GIST (median, 56 years). There is some female predominance for NF1-associated GIST, in contrast to a weak male predominance for patients with sporadic GIST. Also in terms of distribution, GIST in NF1 occur predominantly in the small intestines, unlike sporadic GIST of which 60% arise in the stomach (4). The occurrence of multiple GIST is notably common in NF1 patients, and it is very uncommon among patients with sporadic GIST (4).
It has been reported that c-KIT activation occurs in all cases of GIST, regardless of the mutational status of KIT (4). In a study by Miettinen et al, no mutations were detected in the genomic DNA of KIT (exons 9, 11, 13, 17) or PDGFRA (exons 12, 18) in NF1 associated GIST, whereas sporadic GIST have a high frequency of such activating mutations (4). In sporadic GIST, these mutations are thought to be central events in tumorigenesis, and their occurrence even in minimal GIST <1 cm in diameter indicates them to be an early pathogenetic event. In regard to KIT mutations, Kinoshita et al. also reported no KIT mutations in 21 GIST in 7 patients with NF1 (such as in our patient described above). Lack of GIST-specific mutations suggests that the pathogenesis of GIST in NF1 patients is different from that of KIT or PDGFRA-driven GIST.
The diagnosis of GIST relies on morphology and immunohistochemistry. In 95% of GIST CD117 is positive (5).
Risk stratification is done on the basis of prognostic factors, which include: mitotic rate, tumor size, tumor site, surgical margins (including whether tumor rupture occurred) (5).
Contrast-enhanced abdominal and pelvic CT-scan is the preferred imaging for staging and follow-up. Recent studies have demonstrated that Response Evaluation Criteria In Solid Tumors (RECIST) is an insensitive tool in evaluating GIST treated with imatinib. Another means of assessment, the Choi criteria, describes a 10% decrease in unidimensional tumor size or a 15% decrease in tumor density on contrast-enhanced CT as an early indicator of response (6). This appears to be more sensitive and more precise than RECIST in assessing the response of GIST to imatinib after 3 months of therapy. This was seen in our case as the patient’s tumor size remained stable after 3 months of imatinib but there was a decrease in tumor density with multiple foci of air seen in the follow up CT scan. So, CT assessment is a sensitive and specific method to assess the response of GIST to imatinib if evaluated by Choi criteria. Evaluation of FDG uptake using PET scan is useful mainly when early detection of tumor response to imatinib treatment is of special concern.
The standard treatment of localized GIST is complete surgical excision, without dissection of clinically negative lymph nodes (5). If complete resection is not feasible, or if cytoreduction is desired to allow less aggressive surgery, initial imatinib pretreatment is recommended (5). Following maximal tumor response, surgery is performed. Mutational analysis may help to exclude less sensitive mutational status (e.g., PDGFRA D842V mutations) from therapy with imatinib. PET scan is particularly useful to assess tumor response very rapidly, so that surgery is not delayed in the case of non-responding disease. In patients with locally advanced or metastatic disease, imatinib is the preferred treatment with the standard dose being 400 mg daily (5). Patients with exon 9 KIT mutations fare better in terms of progression free survival on higher doses, i.e. 800 mg daily, which is therefore standard treatment in this subgroup. Treatment should be continued indefinitely since treatment interruption is generally followed by rapid tumor progression. Close monitoring of tumor response should be continued throughout treatment, since the risk of secondary progression persists over time. The standard approach in the case of tumor progression is to increase the imatinib dose to 800 mg daily. In case of progression or intolerance on imatinib, the second-line standard treatment is sunitinib. This drug was proved effective in improving progression free survival following a ‘4 weeks on -2 weeks off’ regimen. After failing on sunitinib, patients with metastatic GIST should be considered for participation in a clinical trial (5).
Conclusions
With the significantly higher incidence of GIST in NF1 patients, we suggest that guidelines be considered to screen for GIST in such patients in order to treat at an earlier stage of the disease. In addition it is important to note that fistula formation between the tumor and the small intestine, as seen in our case, is a possible complication of tyrosine kinase inhibitors. There is one reported case of vesicocutaneous fistula formation (7) and another reported case of colonic perforation (8) both during treatment with sunitinib. Clinicians need to be alert for this complication while treating GIST with tyrosine kinase inhibitors.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 2002;347:472-80. [PubMed]
- Barros A, Linhares E, Valadão M, et al. Extragastrointestinal stromal tumors (EGIST): a series of case reports. Hepatogastroenterology 2011;58:865-8. [PubMed]
- Goh BK, Chow PK, Kesavan SM, et al. A single-institution experience with eight CD117-positive primary extragastrointestinal stromal tumors: critical appraisal and a comparison with their gastrointestinal counterparts. J Gastrointest Surg 2009;13:1094-8. [PubMed]
- Miettinen M, Fetsch JF, Sobin LH, et al. Gastrointestinal stromal tumors in patients with neurofibromatosis 1: a clinicopathologic and molecular genetic study of 45 cases. Am J Surg Pathol 2006;30:90-6. [PubMed]
- Casali PG, Jost L, Reichardt P, et al. Gastrointestinal stromal tumours: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol 2009;20:64-7. [PubMed]
- Benjamin RS, Choi H, Macapinlac HA, et al. We should desist using RECIST, at least in GIST. J Clin Oncol 2007;25:1760-4. [PubMed]
- Watanabe K, Otsu S, Morinaga R, et al. Vesicocutaneous fistula formation during treatment with sunitinib malate: Case report. BMC Gastroenterol 2010;10:128. [PubMed]
- Hur H, Park AR, Jee SB, et al. Perforation of the colon by invading recurrent gastrointestinal stromal tumors during sunitinib treatment. World J Gastroenterol 2008;14:6096-9. [PubMed]