Local excision for early rectal cancer: transanal endoscopic microsurgery and beyond
Introduction
Colorectal cancer (CRC) is the third most common cancer diagnosis and the third leading cause of cancer death in the United States (US). With the increase in population screening, the overall incidence of CRC in the US has decreased (1). Furthermore, there has been an increase in the detection of early stage CRC. In 2013, the American Cancer Society reported data from the National Cancer Institute indicating that approximately 40% of all CRC are early stage cancers (1). Early stage cancer is associated with higher (~90%) 5-year survival. Early stage CRC is defined as lesions limited to the bowel wall with no disease extension beyond the submucosa (T1) or the muscularis mucosa (T2). Furthermore, there is no evidence of lymph node spread (N0).
The management of early stage CRC, in particular rectal cancer, can be challenging. Traditionally, treatment has involved major radical abdominal surgery known as the total mesorectal excision (TME) with the potential for a temporary or permanent stoma. The aim of this procedure is to achieve adequate tumor clearance through the removal of the primary tumor including the mesorectum with the associated regional lymph nodes (2-4). TME or radical surgery is the primary surgery that offers excellent rates of local control and therefore, excellent long-term survival. Patients who undergo radical surgery for stage I and II rectal cancer can expect excellent long-term results which approach 4.5% 5-year local recurrence rates and 90% 5-year disease free survival (DFS) rates (5). However, the morbidity is high (30-68%) with a mortality that approaches 7% (2,5-7). Radical surgery is often followed by significant complications including anastomotic leakage, sepsis, permanent or temporary stoma, perineal wound complications, and urinary, sexual and bowel dysfunction that may diminish quality of life (2,3,5-9).
Given these significant complications, there has been increased interest in the locoregional treatment of early rectal cancer, as some patients may be cured by avoidance of radical surgery and its concomitant disadvantages (10,11). Local excision (LE) of early rectal cancer is an attractive alternative to radical surgery for several reasons. First, the surgery is less invasive and associated with less postoperative pain and a shorter length of stay. The surgery preserves normal bowel function without the use of a stoma. There is less associated perioperative morbidity. Furthermore, newer methods known as transanal endoscopic microsurgery (TEM) or transanal minimally invasive surgery (TAMIS) have been introduced that provide better visualization of tumors in the mid and upper rectum. The aim of this review is to guide the reader in the understanding of the current debates in the management of early stage rectal cancer. This review will include a discussion of patient selection, surgical techniques, and expected oncological outcomes following treatment.
Patient selection
Strict patient selection for LE, together with full-thickness and margin-free excision is crucial for patient outcomes (12). In carefully selected patients local recurrence rates have been reported to be <4% and LE can be curative, with similar oncological outcomes to radical surgery (10). There are several variables that must be evaluated when considering a patient for LE. The key variables include the following characteristics of the tumor: differentiation, the presence of lymphovascular invasion (LVI), the location in the rectum, the size, and the clinical stage. Other key variables that are important to consider prior to performing surgery for rectal cancer are the characteristics of the patient that may put him or her at a higher surgical risk.
To properly select the patients that will benefit from LE, first, digital rectal exam is performed which may determine the mobility of the tumor, the distance from anal verge, and the strength of the anal sphincter. Further, proctoscopy will help in examining more proximal tumors for size and distance from the anal verge. In general, LE can be technically performed for tumors that occupy no more than 30% of the bowel circumference, are no larger than 3 cm in size, and are mobile.
The best method for clinical staging of rectal cancer remains a controversial topic among health care providers. Preoperative identification of tumor depth of invasion (T stage) in the rectal wall and lymph nodes (N stage) can be a challenge. Both modern imaging modalities of endorectal ultrasound (ERUS) and magnetic resonance imaging (MRI) have been used to detect depth of tumor invasion and lymph nodes metastases in rectal cancer (3,10). The reported sensitivity and specificity of ERUS for depth of tumor invasion, perirectal tissue invasion and lymph node involvement is 94%, 90% and 67%, and 86%, 75% and 78%, respectively (13). The major disadvantage of ERUS is the variability in the interpretation of the study due to its dependence on one individual to perform and read the study accurately. MRI has a sensitivity and specificity for T staging ranging from 85% to 100% and from 91% to 98%, respectively (14,15). MRI is also superior at mesorectal lymph node staging with similar sensitivity and specificity as T staging (16). Both imaging modalities will not determine the absence of occult nodal metastases with complete certainty, and some authors suggest that both modalities can be used in combination to increase the likelihood of accurate local staging (3,17).
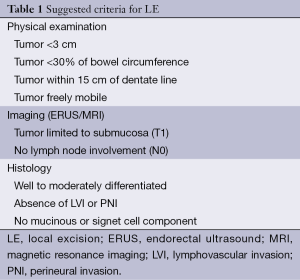
Histological evaluation of the initial endoscopic biopsy of a rectal tumor may aid in determining tumors at a higher risk of lymphatic spread. Important histopathological indicators of aggressive tumor behavior include: histological grade, mucinous tumors, signet cell tumors, and the presence of LVI or perineural invasion (PNI) (Table 1) (18,19). Though controversial, tumor histologic grade is considered a stage-independent prognostic indicator and poorly differentiated colorectal adenocarcinoma is associated with worse patient survival (20-22). Mucinous adenocarcinoma is defined by the findings of >50% of the tumor volume composed of extracellular mucin. These tumors are frequently associated with hereditary non-polyposis CRC (HNPCC) and have the potential to behave more aggressively especially if the tumor is found to be microsatellite stable (23,24). Signet ring adenocarcinoma occurs in less than 1% of patients with colorectal adenocarcinoma. By definition this tumor is poorly differentiated and carries a worse outcome than conventional adenocarcinoma (24-26). Several authors have identified both PNI and LVI as being poor predictors for survival both in those patients treated with multimodality therapy and those treated with surgery alone. Cienfuegos et al. demonstrated a nearly 4-fold risk of recurrence in patient following neoadjuvant therapy for rectal cancer with PNI or LVI. Furthermore PNI and LVI have been shown to be independent predictive variables for poor survival (27). For this reason, many support more radical surgery in this cohort of patients.
Full table
Traditionally, only rectal cancer below 10 cm was considered a candidate for LE. This was due to the limitation of the surgeons’ ability to reach higher and the lack of proper visualization of the rectal tumor. With advances in technology and instrumentation, tumors that are higher up can be reached with good visualization. Newer methods including TEM and TAMIS may allow access up to 15 cm in the rectum. It is important that the patient is aware that these procedures will most likely result in a perforation of the bowel above the retroperitoneum and into the peritoneal cavity which will require repair. The details of these procedures are discussed further in this review.
Extended indications for LE have been reported. Currently, patients with a clinical stage ≥T2 rectal adenocarcinoma should undergo radical surgery. Patients with a diagnosis of more advanced rectal cancer who are not candidates for radical surgery due to high operative risk or those who refuse to undergo radical surgery may be considered for neoadjuvant therapy followed by LE of residual disease (28). Furthermore, the use of LE in patients with early rectal cancer treated with neoadjuvant therapy has been studied in clinical trials with mixed results (29-31). Currently, there is limited data supporting LE or close observation in those patients with a complete clinical response following neoadjuvant therapy as an alternative to radical surgery (5,7,10).
Surgical methods of local excision (LE)
Transanal excision (TAE)
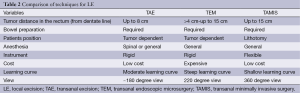
Tumors that are less than 10 cm from the anal verge can be resected with a TAE. In preparation for surgery, a full bowel prep is prescribed, systemic antibiotics are administered, and all anticoagulant use is discontinued. Positioning in the operating room is dependent on the location of the tumor. The patient is placed in lithotomy position for posterior tumors and in prone jackknife for anterior and lateral tumors. Regional or general anesthesia can be utilized to remove the tumor (Table 2). To aid in visualization, the anus is gently dilated and retracted with a Lone Star® (32). The goal of TAE is a full thickness excision of the tumor down to the mesorectal fat with at least 1 cm radial/circumferential margin. In anterior tumors that abut the posterior vaginal wall, this may not be possible and a partial excision is then carried out. Good hemostasis is obtained and the defect in the bowel wall is closed in a transverse manner to avoid narrowing the lumen using interrupted absorbable sutures. The specimen should be oriented by the surgeon for pathological assessment of the margins. Postoperatively, patients experience minimal pain but fever is not uncommon. Patients can resume regular diet and activity within 24 hours (33). Postoperative complications are infrequent and include rectal bleeding which is the most common (6%), rectal stenosis (5.5%), urinary retention (1.5%), fecal incontinence (0.5%), and rectovaginal fistula (<1%) (34,35). If patients receive radiation prior to resection, rectal pain is the most common complication (8%) (36).
Full table
The major disadvantage for TAE is the poorer surgical outcomes. Moore and others have demonstrated that newer procedures such as TEM yields clear margins more frequently than with the traditional TAE (90% vs. 71%) and significant less chance of tumor fragmentation, 94% vs. 65% respectively (37). Intraoperative suboptimal visualization has been hypothesized as the cause for the increase risk of positive margins and tumor fragmentation following TAE (34).
Transanal endoscopic microsurgery (TEM)
TEM was first introduced in 1980’s by Beuss as an alternative to radical surgery for the removal of rectal polyps. The TEM system consists of a dedicated beveled rectoscope with a 4.5 cm diameter and a maximum distance of 200 mm. This scope is placed in the anus forming an airtight seal to allow for insufflation of the rectum and greatly aiding in visualization (11,38,39). The view is magnified and approximately 220 degrees of the rectum can be seen at once. In preparation for surgery, a full bowel prep is prescribed, systemic antibiotics are administered, and all anticoagulant use is discontinued. Anesthesia is provided with either spinal or general and the patient is positioned on the operating room table so the tumor is in the dependent position (Table 2) (32,40). The resectoscope allows access to more proximal rectal lesions up to 15 cm. Because the distal rectum will form the seal with the resectoscope, very low tumors (<5 cm from the anal verge) are not visualized adequately with the TEM procedure. The rectum is insufflated with a standard laparoscopic CO2 insufflator, and then a full thickness excision is performed using laparoscopic instruments to achieve a 1 cm circumferential margin (32,33). The bowel wall defect is closed transversely, and the specimen oriented for pathological review. If the tumor is above the peritoneal reflection, the abdominal cavity may be perforated and this may require a laparotomy to repair (33). Postoperatively, patients are expected to have an overnight hospital stay and quick recovery with early resumption of normal diet and activities (32,33).
The conversion rate from TEM to radical surgery from an abdominal approach has been reported to be 4.3% in one large series of 693 patients (41). The most common complications reported are hemorrhage (27%), urinary tract infection (21%), and suture line dehiscence (14%) (41). Bleeding and perforation can become life threatening especially in multimorbid or elderly patients. They frequently require reoperations and extend hospital stays (42-44). The reported incidence of fecal incontinence developing after insertion of the resectoscope is 1% and this is generally temporary (41).
The major disadvantage to the TEM procedure which has resulted in a slow adoption in the US is the expense of the resectoscope. Although it clearly demonstrates better visualization, it has a very limited clinical role to smaller tumors in the rectum located from 5 to 15 cm. Another disadvantage of TEM is the steep learning curve that is associated with its use. Barendse et al. demonstrated by observing four different providers resect 693 lesions with TEM that a significant learning curve was associated with lowering conversion rates, peritoneal entrance, and procedure time (41). This same study also demonstrated that in patients undergoing TEM after the surgeon had performed at least 35 procedures, the risk of recurrence for malignant lesions declined by 10% as compared to those individuals undergoing surgery in the first 1-35 procedures (41).
Transanal minimally invasive surgery (TAMIS)
TAMIS was first described in 2009 as an alternative to the more expensive system for TEM. The “Tamis platform” uses any of the several available single incision laparoscopy surgery (SILS) ports. By using this port, conventional laparoscopic instrumentation including the camera can be used to perform the procedure. In preparation for surgery, a full bowel prep is prescribed, systemic antibiotics are administered, and all anticoagulant use is discontinued. Anesthesia is provided with either spinal or general and the patient is placed in the dorsal lithotomy position (Table 2). A SILS port is first lubricated and introduced into the anal canal and pneumorectum is established with a standard laparoscopic CO2 insufflator (45,46). Laparoscopic camera lens (preferably using a 5-mm 30 degree or 45 degree lens) and instruments such as graspers, thermal energy devices, and needle drives are introduced through the SILS port to assist the operator in performing a full-thickness resection of the neoplasm with 1 cm margins. The remaining rectal defect is closed in the transverse direction and the specimen oriented for pathological review (46). If the tumor is above the peritoneal reflection, the abdominal cavity may be perforated and this may require laparotomy to repair (33). Postoperatively, patients are expected to have an overnight hospital stay and quick recovery with early resumption of normal diet and activities. Several investigators are designing the TAMIS platform so that the procedure can be performed with the assistance of the Da Vinci® robot.
Complications following the TAMIS procedure are infrequent with an overall rate of 7.4% (45). The conversion rate in 390 cases performed for both benign and malignant lesions was 2.3% (45). Inadvertent peritoneal entry during TAMIS was reported in 1% of cases and in some cases, the closure of the rectum was successful transanally (45). In malignant polyps, the rate of positive margins was 4.4% and the rate of tumor fragmentation was 4.1% (45).
Oncological outcomes from LE
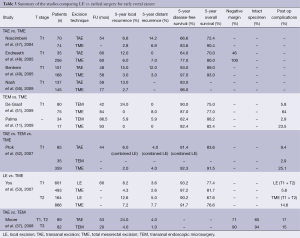
The advances in the management of rectal cancer have risen from a desire by those who take care of these patients to improve oncological outcomes while maintaining good quality of life. This desire has been the leading force for the development of newer surgical methods which are less invasive. Colorectal surgery is one of the leading specialties in minimally invasive and robotic surgery techniques and the desire to expand the role of LE follows naturally. Early results from studies examining LE for rectal cancer have been mixed (Table 3). For this reason, TAE became a procedure reserved for benign lesions. Presently, only clinically staged T1 rectal tumors with favorable histopathology are considered eligible for LE alone without multimodality therapy (54-58).
Full table
Interest in developing newer procedures for LE of rectal tumors was driven by the findings of high recurrence rates seen after transanal resection of benign and malignant lesions. Pigot et al. demonstrated that in large rectal tumor up to 6 cm, the risk or recurrence of benign polyps was 10% (34). If a malignancy was identified, the risk of recurrence was 20%. Others have reported local recurrence rates up to 39% (59-63). Pigot further speculated that the results from TAE can be explained by inadequate intraoperative exposure and suggested that the newer and improved techniques of LE may improve outcomes (34).
Several single series have been published demonstrating superiority of new procedures such as TEM or TAMIS over TAE with regards to margin of resection and tumor fragmentation. Baatrup et al. examined his series of 143 consecutive TEM resections for rectal cancer. Of the patients that were pathological stage T1 tumors, the local recurrence rate was 12% (64). He also found that the significant predictors for survival in his group of patients were tumor size and patient age. He strongly urged that tumors greater than 3 cm should not be removed by LE. In a similar study by Lezoche et al., 135 patients were followed who underwent TEM (65). There were no local recurrences noted in patients with pathological stage T1 tumors and the overall survival rate was 86% at 193 months. Moore et al. in 2007 reported a retrospective comparison of TEM to TAE for rectal cancer (37). In this study, 171 patients (82 with TEM) were analyzed. This study included equal number of patients in each group with T2 and T3 tumors. Patients undergoing TEM had an overall lower recurrence rate (8%) when compared to patients undergoing TAE (24%) but this did not reach statistical significance.
When comparing the results of LE to radical surgery, local recurrence rates tend to be higher for both T1 (8.2-23%) and T2 adenocarcinomas (13-30%) undergoing LE when compared to radical surgery for T1-T2 disease (3-7.2%) (36,49,53,66). However, in the studies evaluating LE there has not been a significant difference in DFS when compared to radical surgery. In patients undergoing LE for T1-T2 disease the DFS at 5 years following LE was 55-93% (36,53). This was comparable to patients undergoing radical surgery whose DFS at 5 years was 77-97% (48,49). The inability to demonstrate improved survival following radial surgery may be due to the retrospective analysis that occurred in many of these studies and the lack of adequate follow up. Only recently has there been an emphasis on appropriate follow up following LE. In addition, Nash et al. emphasizes from his review of this topic that when he analyzed the patients he followed after LE, there was a survival difference seen between LE and radical surgery and this difference was the result of longer follow up (50). He noted a significantly increased rate of cancer-related death at 4-8 years following LE when compared to radical surgery. He recommend that all patients undergoing LE be committed to long term follow-up.
Whether LE compromises the oncological outcome with the risk of recurrence and local failure remains unknown. Lymph node metastasis occurs in 0-12% in T1 and 10-22% in T2 rectal cancer, however, as local lymph nodes are not sampled using TEM, it is reliant on preoperative staging and histopathological features of the tumor to direct further adjuvant treatment (3,67,68). Comparing different LE techniques; the negative margin is most likely achieved with TEM compared to TAE (64,65). Furthermore, the local recurrence rate is lower with TEM compared to TAE (37). This is likely the direct result of improved visibility that is achieved with TEM (69) Whether or not these differences ultimately affect DFS is yet to be determined.
Radical resection immediately after LE
Due to the variability in the sensitivity and specificity of the preoperative staging modalities, it is not uncommon for a preoperatively staged T1N0 rectal cancer to have a final pathological stage of T2 or T3. Moreover; a positive margin following LE carries a high risk of recurrence (68). One method of managing unfavorable pathology is to offer the patient immediate radical surgery. Hahnloser et al. reported his experience at Mayo clinic with immediate radical resection after LE of rectal cancer (70). In this series, 52 patients underwent radical surgery within 30 days after LE were matched with 90 patients with a T2-3N0-1 primary as a radical surgery control group. The indications for radical re-resection were: cancerous polyp, positive margins, LVI, advanced stage, nodal disease and residual cancer. The five-year overall survival for the study cases vs. the control case was (79% vs. 91%), respectively and the ten-year survival was (65% vs. 78%), respectively with no statistical significant.
Several studies have reported that the oncologic outcomes in patients treated by immediate radical surgery after LE for unfavorable histologic findings are comparable to that of radical surgery performed as a primary treatment (2,10,33,70). However, there is no consensus on the timing of radical surgery or on the use of radiotherapy before radical surgery (9).
LE following neoadjuvant therapy
Excellent response to neoadjuvant therapy for rectal cancer has been observed with complete tumor regression even for advance clinical stages in 10% to 30% of patients (10,71,72). These finding have translated into a significant reduction in local recurrence rates from 12% to 4% (73). In patients with pathological complete response (pCR), the risk of lymph node involvement is 1.8% compared to 24-52% in those who didn’t have pCR (9). Furthermore, patients with a pCR tend to have favorable long-term outcomes, including better overall survival and lower recurrence rates (9,74,75). This had led some clinician to question the need for radical surgery with its associated morbidity in those who have a clinically complete response (cCR) confirmed by endoscopic exam.
Habr-Gama et al. compared the long term outcomes between patients who were found to have incomplete clinical response (iCR) and underwent radical surgery with patients who had cCR and underwent a “watch and wait” approach (30). In this series, a total of 265 patients with T2-4 rectal adenocarcinoma received neoadjuvant chemoradiotherapy (CRT). A total of 71 (26.8%) had cCR and underwent watch and wait approach and 194 (73.2%) had iCR and underwent radical resection. At resection, 22 (8.3%) were found to have pCR on the resection specimen. The five-year overall and DFS was 100% and 92% in the watch and wait group and 88% and 83% in the radical resection group respectively. In addition, Perez et al. reported on 15 patients with clinical stage T2N0 rectal cancer who underwent neoadjuvant therapy (31). Therapy was followed by “watch and wait” if a cCR occurred, TEM was performed for a partial response with minimal residual disease, and radical surgery was performed for non-responders. The findings from this study demonstrated that for T2N0 tumors, if a cCR to neoadjuvant therapy does not occur, this appears to be a poor prognostic indicator for unfavorable pathological features as nearly 70% of these patients had ypT2 or ypT3 features and those patients are not ideal candidates for LE.
Currently, the standard of care for T2 rectal adenocarcinoma is radical surgery to ensure accurate staging and decrease the risk of local recurrence but with the promising results of pCR; extended indications for LE have been considered as a middle ground between radical surgery and observation in good responders. The American College of Surgeons Oncology Group (ACOSOG) completed a prospective phase II trial that examined the efficacy and safety of neoadjuvant chemoradiotherapy and LE for T2N0 rectal cancer (76). A total of 77 patients who underwent neoadjuvant therapy and LE were included in the analysis. The pCR rate was 44% and tumor downstaging occurred in 64% of patients. The rate of margin positivity at the time of resection approached 0%. However, 39% of patients developed CRT-related grade ≥3 complications and the trial was closed early. Therefore, long-term survival data is not available, presently. Belluco et al. compared patients with T3N0-1M0 mid and distal rectal adenocarcinoma who underwent TME or LE and were found to have a pCR (74). A total of 139 patients were included and 110 (93%) underwent TME and 29 (17%) underwent LE, 42 (30.2%) were found to have a pCR. In follow up of 55.4 months, there was no difference in the local recurrence between radical surgery vs. LE. Currently, although neoadjuvant therapy may benefit some patients with early stage rectal cancer, indiscriminate use is not recommended in this population owing to the overtreatment of the majority (36).
Adjuvant therapy following LE
In an attempts to improve the oncological outcome and decrease recurrence; adjuvant therapy has been given following LE. To examine the efficacy of this approach, the Cancer and Leukemia Group B (CALGB) has performed a prospective, multi-institutional study on patients with T1 and T2 distal rectal cancer treated with LE with and without adjuvant therapy (77). In this study, 59 patients with T1 tumor were treated with LE alone and 51 patients with T2 tumor were treated with LE followed by adjuvant CRT. The median follow up was 7 years. The ten-year overall survival and DFS were 84% and 75% for T1, and 66% and 64% for T2 respectively. The local recurrence and distant failure rates for T1 tumors were 8% and 5%, while T2 tumors were 18% and 12% respectively. This results show that T2 tumors had a higher rate of recurrence and shorter overall and DFS even with the administration of adjuvant CRT when compared to T1 or historic radical resection. Therefore, adjuvant CRT following LE maybe reserved for patients with high risk pathology who are unfit to undergo radical resection.
Surveillance following LE
Surveillance guidelines published by the National Comprehensive Cancer Network (NCCN) following LE for T1 rectal cancer include the following: (I) a complete history and physical exam every 3-6 months for 2 years, then every 6 months for a total of 5 years; (II) CEA every 3-6 months for 2 years; (III) chest, abdomen, and pelvic computerized tomogram annually for 3 years; (IV) colonoscopy at one year and thereafter depending on findings; (V) proctoscopy every 6 months for 5 years (78). However, as stated early, others have demonstrated a benefit in follow up for up to 9 years following LE (67).
Conclusions
Historically, oncological outcomes from the use of LE for the treatment of early rectal cancer have been disappointing. However, in carefully selected patients with early (T1) rectal cancer, LE by means of the newer methods of TEM and TAMIS is a promising alternative to radical surgery with minimal morbidity and acceptable oncological outcomes. Currently, there are minimal studies evaluating combined use of neoadjuvant therapy and LE for ≥ T2 lesions which limits its generalizability. Furthermore, several authors are supporting no surgery with a “watch and wait” approach for patients with a cCR because the oncological outcomes are no different than radical surgery. Further prospective clinical trials are needed to determine the most promising roles for LE in the management of rectal cancer.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Colorectal Cancer Facts & Figures 2014-2016. Atlanta, GA: American Cancer Society, 2014.
- Elmessiry MM, Van Koughnett JA, Maya A, et al. Local excision of T1 and T2 rectal cancer: proceed with caution. Colorectal Dis 2014;16:703-9. [PubMed]
- Ung L, Chua TC, Engel AF. A systematic review of local excision combined with chemoradiotherapy for early rectal cancer. Colorectal Dis 2014;16:502-15. [PubMed]
- Heald RJ, Moran BJ, Ryall RD, et al. Rectal cancer: the Basingstoke experience of total mesorectal excision, 1978-1997. Arch Surg 1998;133:894-9. [PubMed]
- Mellgren A, Sirivongs P, Rothenberger DA, et al. Is local excision adequate therapy for early rectal cancer? Dis Colon Rectum 2000;43:1064-71; discussion 1071-4. [PubMed]
- Nelson H, Sargent DJ. Refining multimodal therapy for rectal cancer. N Engl J Med 2001;345:690-2. [PubMed]
- Peeters KC, van de Velde CJ, Leer JW, et al. Late side effects of short-course preoperative radiotherapy combined with total mesorectal excision for rectal cancer: increased bowel dysfunction in irradiated patients--a Dutch colorectal cancer group study. J Clin Oncol 2005;23:6199-206. [PubMed]
- Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 1986;1:1479-82. [PubMed]
- Nesbakken A, Nygaard K, Bull-Njaa T, et al. Bladder and sexual dysfunction after mesorectal excision for rectal cancer. Br J Surg 2000;87:206-10. [PubMed]
- Hompes R, Cunningham C. Extending the role of Transanal Endoscopic Microsurgery (TEM) in rectal cancer. Colorectal Dis 2011;13 Suppl 7:32-6. [PubMed]
- Palma P, Horisberger K, Joos A, et al. Local excision of early rectal cancer: is transanal endoscopic microsurgery an alternative to radical surgery? Rev Esp Enferm Dig 2009;101:172-8. [PubMed]
- Kwok H, Bissett IP, Hill GL. Preoperative staging of rectal cancer. Int J Colorectal Dis 2000;15:9-20. [PubMed]
- Bipat S, Glas AS, Slors FJ, et al. Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging--a meta-analysis. Radiology 2004;232:773-83. [PubMed]
- Brown G, Richards CJ, Bourne MW, et al. Morphologic predictors of lymph node status in rectal cancer with use of high-spatial-resolution MR imaging with histopathologic comparison. Radiology 2003;227:371-7. [PubMed]
- Kim JH, Beets GL, Kim MJ, et al. High-resolution MR imaging for nodal staging in rectal cancer: are there any criteria in addition to the size? Eur J Radiol 2004;52:78-83. [PubMed]
- Glasgow SC. Advancing Dr Wong's vision for evaluating rectal cancer. Dis Colon Rectum 2013;56:1325-6. [PubMed]
- Muthusamy VR, Chang KJ. Optimal methods for staging rectal cancer. Clin Cancer Res 2007;13:6877s-84s. [PubMed]
- Saraste D, Gunnarsson U, Janson M. Predicting lymph node metastases in early rectal cancer. Eur J Cancer 2013;49:1104-8. [PubMed]
- Stitzenberg KB, Sanoff HK, Penn DC, et al. Practice patterns and long-term survival for early-stage rectal cancer. J Clin Oncol 2013;31:4276-82. [PubMed]
- Blenkinsopp WK, Stewart-Brown S, Blesovsky L, et al. Histopathology reporting in large bowel cancer. J Clin Pathol 1981;34:509-13. [PubMed]
- Jass JR, Atkin WS, Cuzick J, et al. The grading of rectal cancer: historical perspectives and a multivariate analysis of 447 cases. Histopathology 1986;10:437-59. [PubMed]
- Compton CC. Pathology report in colon cancer: what is prognostically important? Dig Dis 1999;17:67-79. [PubMed]
- Verhulst J, Ferdinande L, Demetter P, et al. Mucinous subtype as prognostic factor in colorectal cancer: a systematic review and meta-analysis. J Clin Pathol 2012;65:381-8. [PubMed]
- Kang H, O'Connell JB, Maggard MA, et al. A 10-year outcomes evaluation of mucinous and signet-ring cell carcinoma of the colon and rectum. Dis Colon Rectum 2005;48:1161-8. [PubMed]
- Chen JS, Hsieh PS, Chiang JM, et al. Clinical outcome of signet ring cell carcinoma and mucinous adenocarcinoma of the colon. Chang Gung Med J 2010;33:51-7. [PubMed]
- Makino T, Tsujinaka T, Mishima H, et al. Primary signet-ring cell carcinoma of the colon and rectum: report of eight cases and review of 154 Japanese cases. Hepatogastroenterology 2006;53:845-9. [PubMed]
- Cienfuegos JA, Rotellar F, Baixauli J, et al. Impact of perineural and lymphovascular invasion on oncological outcomes in rectal cancer treated with neoadjuvant chemoradiotherapy and surgery. Ann Surg Oncol 2015;22:916-23. [PubMed]
- Tsai BM, Finne CO, Nordenstam JF, et al. Transanal endoscopic microsurgery resection of rectal tumors: outcomes and recommendations. Dis Colon Rectum 2010;53:16-23. [PubMed]
- Hughes R, Harrison M, Glynne-Jones R. Could a wait and see policy be justified in T3/4 rectal cancers after chemo-radiotherapy? Acta Oncol 2010;49:378-81. [PubMed]
- Habr-Gama A, Gama-Rodrigues J, São Julião GP, et al. Local recurrence after complete clinical response and watch and wait in rectal cancer after neoadjuvant chemoradiation: impact of salvage therapy on local disease control. Int J Radiat Oncol Biol Phys 2014;88:822-8. [PubMed]
- Perez RO, Habr-Gama A, São Julião GP, et al. Transanal local excision for distal rectal cancer and incomplete response to neoadjuvant chemoradiation - does baseline staging matter? Dis Colon Rectum 2014;57:1253-9. [PubMed]
- Papagrigoriadis S. Transanal endoscopic micro-surgery (TEMS) for the management of large or sessile rectal adenomas: a review of the technique and indications. Int Semin Surg Oncol 2006;3:13. [PubMed]
- Heafner TA, Glasgow SC. A critical review of the role of local excision in the treatment of early (T1 and T2) rectal tumors. J Gastrointest Oncol 2014;5:345-52. [PubMed]
- Pigot F, Bouchard D, Mortaji M, et al. Local excision of large rectal villous adenomas: long-term results. Dis Colon Rectum 2003;46:1345-50. [PubMed]
- Piccinini EE, Ugolini G, Rosati G, et al. Transanal local resection for benign and malignant rectal tumours. Int J Colorectal Dis 1995;10:112-6. [PubMed]
- Garcia-Aguilar J, Mellgren A, Sirivongs P, et al. Local excision of rectal cancer without adjuvant therapy: a word of caution. Ann Surg 2000;231:345-51. [PubMed]
- Moore JS, Cataldo PA, Osler T, et al. Transanal endoscopic microsurgery is more effective than traditional transanal excision for resection of rectal masses. Dis Colon Rectum 2008;51:1026-30; discussion 1030-1. [PubMed]
- Buess G, Mentges B, Manncke K, et al. Technique and results of transanal endoscopic microsurgery in early rectal cancer. Am J Surg 1992;163:63-9; discussion 69-70. [PubMed]
- Zoller S, Joos A, Dinter D, et al. Retrorectal tumors: excision by transanal endoscopic microsurgery. Rev Esp Enferm Dig 2007;99:547-50. [PubMed]
- Atallah SB, Albert MR. Transanal minimally invasive surgery (TAMIS) versus transanal endoscopic microsurgery (TEM): is one better than the other? Surg Endosc 2013;27:4750-1. [PubMed]
- Barendse RM, Dijkgraaf MG, Rolf UR, et al. Colorectal surgeons' learning curve of transanal endoscopic microsurgery. Surg Endosc 2013;27:3591-602. [PubMed]
- Kreissler-Haag D, Schuld J, Lindemann W, et al. Complications after transanal endoscopic microsurgical resection correlate with location of rectal neoplasms. Surg Endosc 2008;22:612-6. [PubMed]
- Featherstone JM, Grabham JA, Fozard JB. Per-anal excision of large, rectal, villous adenomas. Dis Colon Rectum 2004;47:86-9. [PubMed]
- Kosciñski T, Malinger S, Drews M. Local excision of rectal carcinoma not-exceeding the muscularis layer. Colorectal Dis 2003;5:159-63. [PubMed]
- Martin-Perez B, Andrade-Ribeiro GD, Hunter L, et al. A systematic review of transanal minimally invasive surgery (TAMIS) from 2010 to 2013. Tech Coloproctol 2014;18:775-88. [PubMed]
- Atallah S, Albert M, Larach S. Transanal minimally invasive surgery: a giant leap forward. Surg Endosc 2010;24:2200-5. [PubMed]
- Nascimbeni R, Nivatvongs S, Larson DR, et al. Long-term survival after local excision for T1 carcinoma of the rectum. Dis Colon Rectum 2004;47:1773-9. [PubMed]
- Endreseth BH, Wibe A, Svinsås M, et al. Postoperative morbidity and recurrence after local excision of rectal adenomas and rectal cancer by transanal endoscopic microsurgery. Colorectal Dis 2005;7:133-7. [PubMed]
- Bentrem DJ, Okabe S, Wong WD, et al. T1 adenocarcinoma of the rectum: transanal excision or radical surgery? Ann Surg 2005;242:472-7; discussion 477-9. [PubMed]
- Nash GM, Weiser MR, Guillem JG, et al. Long-term survival after transanal excision of T1 rectal cancer. Dis Colon Rectum 2009;52:577-82. [PubMed]
- De Graaf EJ, Doornebosch PG, Tollenaar RA, et al. Transanal endoscopic microsurgery versus total mesorectal excision of T1 rectal adenocarcinomas with curative intention. Eur J Surg Oncol 2009;35:1280-5. [PubMed]
- Ptok H, Marusch F, Meyer F, et al. Oncological outcome of local vs radical resection of low-risk pT1 rectal cancer. Arch Surg 2007;142:649-55; discussion 656. [PubMed]
- You YN, Baxter NN, Stewart A, et al. Is the increasing rate of local excision for stage I rectal cancer in the United States justified?: a nationwide cohort study from the National Cancer Database. Ann Surg 2007;245:726-33. [PubMed]
- Heintz A, Mörschel M, Junginger T. Comparison of results after transanal endoscopic microsurgery and radical resection for T1 carcinoma of the rectum. Surg Endosc 1998;12:1145-8. [PubMed]
- Palma P, Freudenberg S, Samel S, et al. Transanal endoscopic microsurgery: indications and results after 100 cases. Colorectal Dis 2004;6:350-5. [PubMed]
- Sengupta S, Tjandra JJ. Local excision of rectal cancer: what is the evidence? Dis Colon Rectum 2001;44:1345-61. [PubMed]
- Varma MG, Rogers SJ, Schrock TR, et al. Local excision of rectal carcinoma. Arch Surg 1999;134:863-7; discussion 867-8. [PubMed]
- Winde G, Nottberg H, Keller R, et al. Surgical cure for early rectal carcinomas (T1). Transanal endoscopic microsurgery vs. anterior resection. Dis Colon Rectum 1996;39:969-76. [PubMed]
- Chiu YS, Spencer RJ. Villous lesions of the colon. Dis Colon Rectum 1978;21:493-5. [PubMed]
- Sakamoto GD, MacKeigan JM, Senagore AJ. Transanal excision of large, rectal villous adenomas. Dis Colon Rectum 1991;34:880-5. [PubMed]
- Nivatvongs S, Nicholson JD, Rothenberger DA, et al. Villous adenomas of the rectum: the accuracy of clinical assessment. Surgery 1980;87:549-51. [PubMed]
- Thomson JP. Treatment of sessile villous and tubulovillous adenomas of the rectum: experience of St. Mark's Hospital. 1963-1972. Dis Colon Rectum 1977;20:467-72. [PubMed]
- Keck JO, Schoetz DJ Jr, Roberts PL, et al. Rectal mucosectomy in the treatment of giant rectal villous tumors. Dis Colon Rectum 1995;38:233-8. [PubMed]
- Baatrup G, Breum B, Qvist N, et al. Transanal endoscopic microsurgery in 143 consecutive patients with rectal adenocarcinoma: results from a Danish multicenter study. Colorectal Dis 2009;11:270-5. [PubMed]
- Lezoche G, Guerrieri M, Baldarelli M, et al. Transanal endoscopic microsurgery for 135 patients with small nonadvanced low rectal cancer (iT1-iT2, iN0): short- and long-term results. Surg Endosc 2011;25:1222-9. [PubMed]
- Madbouly KM, Remzi FH, Erkek BA, et al. Recurrence after transanal excision of T1 rectal cancer: should we be concerned? Dis Colon Rectum 2005;48:711-9; discussion 719-21. [PubMed]
- Zenni GC, Abraham K, Harford FJ, et al. Characteristics of rectal carcinomas that predict the presence of lymph node metastases: implications for patient selection for local therapy. J Surg Oncol 1998;67:99-103. [PubMed]
- Paty PB, Nash GM, Baron P, et al. Long-term results of local excision for rectal cancer. Ann Surg 2002;236:522-29; discussion 529-30. [PubMed]
- McLemore EC, Coker A, Jacobsen G, et al. eTAMIS: endoscopic visualization for transanal minimally invasive surgery. Surg Endosc 2013;27:1842-5. [PubMed]
- Hahnloser D, Wolff BG, Larson DW, et al. Immediate radical resection after local excision of rectal cancer: an oncologic compromise? Dis Colon Rectum 2005;48:429-37. [PubMed]
- Habr-Gama A, de Souza PM, Ribeiro U Jr, et al. Low rectal cancer: impact of radiation and chemotherapy on surgical treatment. Dis Colon Rectum 1998;41:1087-96. [PubMed]
- Hiotis SP, Weber SM, Cohen AM, et al. Assessing the predictive value of clinical complete response to neoadjuvant therapy for rectal cancer: an analysis of 488 patients. J Am Coll Surg 2002;194:131-5; discussion 135-6. [PubMed]
- Folkesson J, Birgisson H, Pahlman L, et al. Swedish Rectal Cancer Trial: long lasting benefits from radiotherapy on survival and local recurrence rate. J Clin Oncol 2005;23:5644-50. [PubMed]
- Belluco C, De Paoli A, Canzonieri V, et al. Long-term outcome of patients with complete pathologic response after neoadjuvant chemoradiation for cT3 rectal cancer: implications for local excision surgical strategies. Ann Surg Oncol 2011;18:3686-93. [PubMed]
- Coco C, Manno A, Mattana C, et al. The role of local excision in rectal cancer after complete response to neoadjuvant treatment. Surg Oncol 2007;16 Suppl 1:S101-4. [PubMed]
- Garcia-Aguilar J, Shi Q, Thomas CR Jr, et al. A phase II trial of neoadjuvant chemoradiation and local excision for T2N0 rectal cancer: preliminary results of the ACOSOG Z6041 trial. Ann Surg Oncol 2012;19:384-91. [PubMed]
- Greenberg JA, Shibata D, Herndon JE 2nd, et al. Local excision of distal rectal cancer: an update of cancer and leukemia group B 8984. Dis Colon Rectum 2008;51:1185-91; discussion 1191-4. [PubMed]
- Engstrom PF, Arnoletti JP, Benson AB 3rd, et al. NCCN Clinical Practice Guidelines in Oncology: rectal cancer. J Natl Compr Canc Netw 2009;7:838-81. [PubMed]


