
A case report of paraneoplastic syndrome in β-hCG-secreting duodenal adenocarcinoma
Introduction
Elevations in serum beta-human chorionic gonadotropin (β-hCG) are usually found in pregnancy or pregnancy-related conditions such as gestational trophoblastic tumors. However, β-hCG can also be produced in the stomach, colon, liver, and lung in small amounts (1). Malignancies in these organs can consequently result in increased serum levels of β-hCG, which is typically a sign of aggressive disease and poor prognosis (2). We report a case of a 50-year-old female newly diagnosed with stage IV duodenal adenocarcinoma with metastasis to the lungs, liver, lymph nodes, and left adrenal gland. Her pregnancy test was initially negative but later in her course changed to positive. Workup including pelvic ultrasound was unrevealing, making intrauterine and ectopic pregnancy unlikely. Her elevated β-hCG was consistent with a paraneoplastic syndrome originating from malignancy of the duodenum, which has not been previously reported on review of literature.
Case presentation
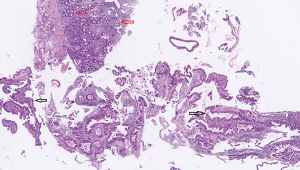
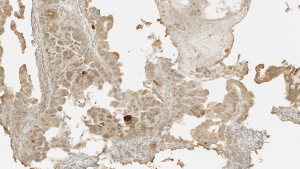
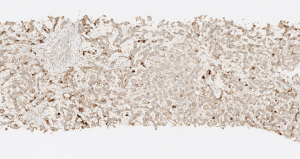
A 50-year-old Hispanic female with multiple liver and lung lesions presented with acute abdominal pain and melena. She had recently been admitted to the hospital with early satiety, intermittent emesis, and unintentional weight loss. This prompted imaging that demonstrated numerous masses in her lungs and liver. After her symptoms improved, she was discharged home with plans for outpatient biopsy until her unintended readmission. Initial urine pregnancy screening was notably negative and the patient was reported to be postmenopausal. Esophagogastroduodenoscopy (EGD) showed extrinsic compression of the gastric antrum and a malignant-appearing duodenal stricture that was biopsied. Pathology revealed infiltrating moderately differentiated papillary and villoglandular adenocarcinoma with extensive lymphatic invasion (Figures 1,2). Molecular studies detected a KRAS mutation in codon 12, 13, but no microsatellite instability nor BRAF mutation. Serum CEA was elevated to 122.9 ng/mL (normal value <3 ng/mL). Subsequent biopsy of one of the liver lesions confirmed metastasis from primary duodenal carcinoma (Figure 3).
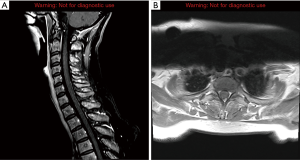
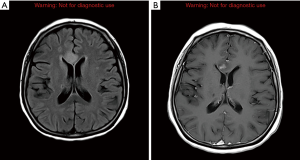
Imaging for cancer staging incidentally revealed bilateral segmental and subsegmental pulmonary emboli and re-demonstrated disseminated disease throughout the lungs, liver, lymph nodes, and left adrenal gland in addition to the duodenal mass. The patient’s symptoms of back pain led to a whole body bone scan that showed focal uptake in the lower cervical spine and left first and second ribs. Magnetic resonance imaging (MRI) of the cervical spine showed enhancement within the right C6 superior articular process as well as possible contrast-enhancing intradural extramedullary nodules suggestive of dural and leptomeningeal metastasis (Figure 4). MRI of the brain demonstrated metastasis within the bilateral frontal and parietal lobes with mild edema and restriction diffusion in the right corpus callosum, which was difficult to delineate between an acute ischemic event and leptomeningeal disease on review by neurologists (Figure 5). Evaluation for potential stroke etiology, including echocardiogram, monitoring for arrhythmias on telemetry, and serum lab markers (lipid profile and hemoglobin A1c), was negative. Cerebrospinal fluid studies showed elevated protein and indeterminate cytology limited by cell degeneration from a traumatic tap. The patient was discharged with plans for repeat brain imaging and initiation of palliative chemotherapy.
Prior to starting cancer treatment, the patient was again readmitted for hyponatremia (sodium level 125) secondary to SIADH and elevated liver function tests. Urine β-hCG was newly positive despite initial negative result. Serum β-hCG was elevated to 17 IU/L (normal value <5 IU/L). The patient’s last menstrual period was about one year ago and she had undergone tubal ligation during her 20s. Pelvic ultrasound was negative for evidence of intrauterine pregnancy or focal mass within the uterus and adnexal regions, with a normal endometrial thickness of 60 mm. Immunohistochemical stainings of both the duodenal and liver biopsies were strongly positive for β-hCG (Figures 2,3). The plan was to initiate mFOLFOX6 + avastin inpatient. Unfortunately, the patient developed worsening altered mental status and decreased responsiveness, and ultimately was transitioned to home hospice after extensive discussion with her husband.
Discussion
Duodenal adenocarcinoma is a rare but aggressive malignancy, representing less than 1% of all gastrointestinal cancers. Patients most often present with abdominal pain and other nonspecific symptoms including nausea, vomiting, fatigue, and weight loss. More advanced disease is associated with bowel obstruction, jaundice, and anemia (3). Metastases to the liver, lungs, and bone are common at presentation, occurring in about 33% of patients. Brain metastasis from small intestine malignancies are uncommon; the largest published case series included only 15 patients with small bowel malignancy metastatic to the brain, including 12 adenocarcinoma and 3 neuroendocrine. Another case series reported a median survival of 10 months in patients with solitary cerebral metastases from primary intestinal malignancies, although they did not differentiate between tumors of the small intestine, colon and rectum (4). Paraneoplastic syndrome from duodenal adenocarcinoma is also exceedingly rare, and there are no published case reports available for review at the time of publication. Paraneoplastic disorders are a heterogenous group of disorders caused by substances released by a tumor or produced in reaction to it. These substances are most commonly hormones or other compounds such as antibodies. Paraneoplastic syndromes alone are uncommon, estimated to affect up to 8% of patients with malignancy and are most commonly associated with small cell lung cancer, gynecologic tumors, breast cancer, and hematologic malignancies. Our patient presented with SIADH, but also was suspected to have paraneoplastic β-hCG production.
hCG is a placental hormone secreted by cells from the implanting conceptus that promotes progesterone production by corpus luteal cells, which in turn supports the endometrial lining and therefore maintains pregnancy. In medical practice, the hormone is primarily used for detection and monitoring of pregnancy, but it is also used as a marker for trophoblastic tumors of placental and germ cell origin (2). Similar to other gonadotropins, hCG is a heterodimeric glycoprotein made up of α and β subunits. The α subunit is common to all glycoprotein hormones whereas the β subunit is unique to each hormone and distinguishes the biological activity (5). β-hCG in particular has been shown to promote cell invasion and tumor growth, as well as to suppress immune responses (6). Although hCG is mainly produced by placental syncytiotrophoblast cells, it is also produced at low levels in other organs. Increased β-hCG serum levels have been shown to occur in various cancers of the GI tract, most commonly in biliary (60%), pancreatic (46%), and gastric cancer (40%), and less frequently in liver (20%) and colorectal cancer (15%) (2) (Table 1).

Full table
Factors associated with worse outcome in duodenal adenocarcinoma include lymph node metastasis, tumor stage, lymphovascular or perineural invasion, and overall cancer stage, with lymph node positivity as one of the most important predictors of decreased survival (3). Serum tumor markers are not frequently used as prognostic indicators and more often monitored as a sign of treatment response. β-hCG production is linked with aggressive behavior in nontrophoblastic tumors and has been found to be associated with poor prognosis in most studies, although others indicate it is of limited prognostic value (2). One report found colorectal cancer patients with normal β-hCG levels had a median survival of 7.9 years compared with 1 year for patients with elevated serum levels, with a statistically significant difference between survival curves in Dukes’ D cancer stage (12). Another study demonstrated 20 colorectal cancer patients with positive β-hCG on both histology and serology had a 5-year survival of 21% versus 62% in the group with no expression in tissue or serum (8). Although β-hCG can be elevated in peri-menopausal stages, our patient initially had a negative pregnancy test that later became positive as her overall clinical condition declined suggestive of increased tumor burden from an aggressive malignancy.
Resection of the primary tumor is the only curative treatment option for duodenal adenocarcinoma; survival benefit has been reported with adjuvant chemotherapy or chemoradiation after curative resection, and with palliative chemotherapy for stage IV disease. Given the similarities between small bowel adenocarcinoma and colorectal cancer, treatment strategies are used interchangeably (3). Current chemotherapy regimens for metastatic stage IV colorectal cancer have shown improved, although modest, survival duration. Given the significant toxicity associated with standard therapy, novel treatment modalities are currently being studied.
Active specific immunotherapy (ASI) has been under investigation with some studies using hCG as a target. Moulton et al., describes a randomized control trial phase II study comprised of 77 patients with metastatic colorectal cancer divided into two vaccine dose groups using a synthetic vaccine targeting the C-terminal peptide of hCG. No significant differences were found in antibody response and survival, however patients who developed anti-hCG antibody levels higher than or equal to the median value exhibited a median survival of 45 weeks compared to 24 weeks for patients who developed levels lower than the median. A different study proposed another vaccine containing a β-hCG mutant designed to eliminate cross-reactivity with luteinizing hormone. Compared to C-terminal β-hCG peptide vaccines, the mutant vaccine resulted in the highest antibody titers, making it a promising therapy in β-hCG-positive cancers (13). Another report by Nand et al. describes a recombinant PiPP monoclonal antibody conjugated to exotoxin that was shown to kill almost 90% of cells in vitro of β-hCG-expressing lymphoma, T-lymphoblastic leukemia, and lung carcinoma (14). Further studies will need to be performed to determine whether ASI can improve overall outcomes of patients with colorectal cancer.
Conclusions
In conclusion, this is the first case of paraneoplastic β-hCG production from a duodenal adenocarcinoma reported in literature. According to some studies serum β-hCG may be an independent prognostic indicator of more aggressive disease and adverse outcomes in colorectal cancer. Investigational studies developing therapies targeting β-hCG are currently underway.
Acknowledgments
The authors would like to thank the family of the patient for allowing us to share the details of her case.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Demirtas E, Krishnamurthy S, Tulandi T. Elevated serum beta-human chorionic gonadotropin in nonpregnant conditions. Obstet Gynecol Surv 2007;62:675-9. [Crossref] [PubMed]
- Stenman UH, Alfthan H, Hotakainen K. Human chorionic gonadotropin in cancer. Clin Biochem 2004;37:549-61. [Crossref] [PubMed]
- Cloyd JM, George E, Visser BC. Duodenal adenocarcinoma: Advances in diagnosis and surgical management. World J Gastrointest Surg 2016;8:212-21. [Crossref] [PubMed]
- Go PH, Klaassen Z, Meadows MC, et al. Gastrointestinal cancer and brain metastasis: a rare and ominous sign. Cancer 2011;117:3630-40. [Crossref] [PubMed]
- Kara E, Dupuy L, Bouillon C, et al. Modulation of Gonadotropins Activity by Antibodies. Front Endocrinol (Lausanne) 2019;10:15. [Crossref] [PubMed]
- Moulton HM, Yoshihara PH, Mason DH, et al. Active specific immunotherapy with a beta-human chorionic gonadotropin peptide vaccine in patients with metastatic colorectal cancer: antibody response is associated with improved survival. Clin Cancer Res 2002;8:2044-51. [PubMed]
- Couvelard A, Paraf F, Vidaud D, et al. Human chorionic gonadotrophin beta expression in malignant Barrett's oesophagus. Virchows Arch 2004;445:279-84. [Crossref] [PubMed]
- Lundin M, Nordling S, Lundin J, et al. Tissue expression of human chorionic gonadotropin beta predicts outcome in colorectal cancer: a comparison with serum expression. Int J Cancer 2001;95:18-22. [Crossref] [PubMed]
- Louhimo J, Alfthan H, Stenman UH, et al. Serum HCG beta and CA 72-4 are stronger prognostic factors than CEA, CA 19-9 and CA 242 in pancreatic cancer. Oncology 2004;66:126-31. [Crossref] [PubMed]
- Murhekar KM, Anuratha JN, Majhi U, et al. Expression of human chorionic gonadotropin beta in gastric carcinoma: A retrospective immunohistochemical study. Indian J Med Paediatr Oncol 2009;30:99-102. [Crossref] [PubMed]
- Alfthan H, Haglund C, Roberts P, et al. Elevation of free beta subunit of human choriogonadotropin and core beta fragment of human choriogonadotropin in the serum and urine of patients with malignant pancreatic and biliary disease. Cancer Res 1992;52:4628-33. [PubMed]
- Carpelan-Holmstrom M, Haglund C, Lundin J, et al. Independent prognostic value of preoperative serum markers CA 242, specific tissue polypeptide antigen and human chorionic gonadotrophin beta, but not of carcinoembryonic antigen or tissue polypeptide antigen in colorectal cancer. Br J Cancer 1996;74:925-9. [Crossref] [PubMed]
- Kvirkvelia N, Chikadze N, Makinde J, et al. Investigation of factors influencing the immunogenicity of hCG as a potential cancer vaccine. Clin Exp Immunol 2018;193:73-83. [Crossref] [PubMed]
- Nand KN, Gupta JC, Panda AK, et al. Development of a recombinant hCG-specific single chain immunotoxin cytotoxic to hCG expressing cancer cells. Protein Expr Purif 2015;106:10-7. [Crossref] [PubMed]


