The clinical significance of simple endoscopic scoring of patients with rectal cancer after concurrent chemoradiotherapy
Introduction
Colorectal cancer (CRC) is one of the most common causes of worldwide cancer death (1). According to the World Health Organization, CRC incidence in Korea is the second highest in the world, with an age-standardized incidence rate of 44.5/100,000 people (2). The standard treatment for locally advanced rectal cancer is neoadjuvant concurrent chemoradiotherapy (CCRT), followed by surgery (3). Neoadjuvant CCRT has proven to be to effective at reducing tumor volume and can facilitate resection with high rate of sphincter preservation (4-6). CCRT also has the potential to increase rates of complete pathologic response (6). A Cochrane review showed that neoadjuvant CCRT in patients with locally advanced rectal cancer reduced local recurrence (7). In their systematic review, they analyzed six randomized controlled trials and found that local recurrence was lower in the CCRT group compared with the radiation-only group. Several studies also reported that neoadjuvant CCRT improves local control and enhances pathologic response but does not improve overall survival (OS) or disease-free survival (DFS) (8-10). Neoadjuvant CCRT is also associated with increased toxicity, including radiation-induced injury and hematologic abnormality, compared with surgery alone (10,11). Recent studies have suggested that the response to neoadjuvant CCRT is associated with long-term outcomes in patients with rectal cancer (12-14). In these studies, higher tumor regression grading after neoadjuvant CCRT was a predictor of favorable long-term outcomes; thus, tumor regression grading was suggested as a surrogate marker for DFS. Regarding prognostic factors, the EORTC 22921 trial suggested that patients whose tumors were down-staged to ypT0–2 were more likely benefit from neoadjuvant CCRT than patients with ypT3–4 staging (15), and several studies found that complete pathologic response was associated with favorable long-term outcomes in patients with locally advanced rectal cancer after neoadjuvant CCRT (6,16). However, few studies have assessed endoscopic findings after neoadjuvant CCRT for predicting long-term outcomes. One study suggested that post-CCRT endoscopic findings were prognostic predictors in patients with rectal cancer; however, the classification of endoscopic findings is overly complicated for clinical application (17).
In this study, we defined a simple endoscopic scoring system to use after neoadjuvant CCRT and evaluated the clinical value of this scoring system for predicting primary-tumor downstaging and the long-term outcomes of patients with rectal cancer after neoadjuvant CCRT.
Methods
Patients and data collection
Between July 2008 and October 2015, medical records from 94 patients with rectal cancer who received CCRT were retrospectively reviewed. Among these patients, those who underwent colonoscopy or sigmoidoscopy after neoadjuvant CCRT were included in the analysis. Patients whose medical records did not include clinicopathological and follow-up data were excluded. Detailed clinical data, including age, sex, tumor location, histopathology, tumor stage, laboratory findings, and endoscopic findings, were collected from medical charts. This study was approved by the Institutional Review Board of Kosin University Gospel Hospital (KUGH 2019-04-006).
Treatment
Neoadjuvant CCRT was determined via conference by a multidisciplinary team. Pre-surgery chemotherapy consisted of a continuous infusion of 5-fluorouracil (425 mg/m2) plus leucovorin (20 mg/m2) on days 1–5 every 4 weeks or capecitabine (825 mg/m2) twice daily on days 1–14 every 3 weeks. Radiotherapy consisted of 50.4 Gy in 28 fractions (180 cGy daily, Monday–Friday), delivered with an energy of 10-MV photons to the primary tumor and to the mesorectal, presacral, and internal iliac lymph nodes via a three-field box technique. All patients were re-staged with computed tomography (CT) prior to surgery, and they proceeded to surgery 5–12 weeks after CCRT. After surgery, all patients received additional chemotherapy consisting of capecitabine or intravenous 5-fluorouracil plus leucovorin or a FOLFOX regimen (combination of oxaliplatin, 5-fluorouracil, and leucovorin).
Classification of endoscopic findings
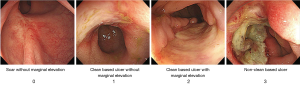
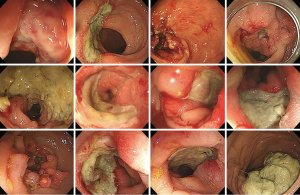
Among the 94 patients with rectal cancer who received CCRT, 41 underwent colonoscopy or sigmoidoscopy after CCRT to evaluate tumor regression. Two experienced gastroenterologists (JH Kim and SJ Park) reviewed all endoscopic findings before and after CCRT and classified the post-CCRT findings into four categories through agreement: score 0= scar without marginal elevation; score 1= clean-based ulcer without marginal elevation; score 2= clean-based ulcer with marginal elevation; score 3= non-clean-based ulcer (Figure 1).
Follow-up surveillance
After adjuvant chemotherapy, patients were seen at an outpatient clinic every 3 months for the first 2 years, then every 6 months for the next 3 years, and then every year after 5 years. Follow-up examination for the first 2 years included carcinoembryonic antigen (CEA) measurement every 3 months and chest and abdominal CT every 6 months; these examinations were performed annually thereafter. Colonoscopy was also performed annually. When recurrence was suspected on radiologic imaging, it was confirmed by endoscopic biopsy or surgery. Local recurrence was defined as any recurrence within the pelvic cavity, and distant recurrence was defined as any recurrence outside the pelvic cavity.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics version 24.0 (IBM Co., Armonk, NY, USA). Student’s t-test and the chi-square test were performed for continuous and categorical variables, as appropriate. A logistic regression analysis was used to assess factors affecting transformation to T stage or N stage after CCRT. A Kaplan-Meier curve for comparing DFS according to scoring was generated, and a log-rank test was used to compare. A Cox proportional-hazards regression model was fit to evaluate factors affecting DFS.
Results
Baseline characteristics
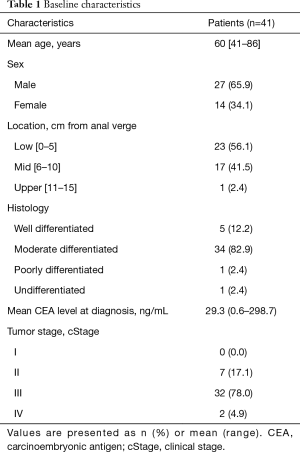
A total of 41 patients was included in the analysis. The median follow-up duration was 55 months (interquartile range: 35–76 months). Mean patient age was 60 years, and 27 patients (65.9%) were male. The mean CEA level at diagnosis was 29.3 ng/mL (range, 0.6–298.7 ng/mL). At diagnosis, 32 patients were clinical stage 3, seven patients were stage 2, and two patients were stage 4. Baseline characteristics are summarized in Table 1.
Full table
Simple endoscopic scoring
We classified post-CCRT endoscopic findings into four categories. Among the 41 patients: score 0, n=1; score 1, n=12; score 2, n=16, score 3, n=12. Initially, we tried to identify the clinical significance of each score’s effect on change to T stage or N stage, and long-term outcomes after CCRT. However, these analyses were not possible because our sample size was too small. Consequently, we divided the patients into two groups: a low-score group (≤2) and a high-score group (3), and we subsequently analyzed clinical significance in both groups.
Post-operative T and N stages according to scoring
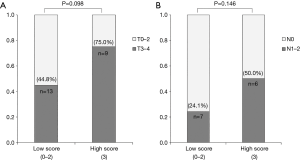
As shown in Figure 2A, 13 of 29 low-score patients (44.8%) had T3 or T4 stage, and 9 of 12 high-score patients (75.0%) had T3 or T4 stage, according to surgical specimen assessment; the difference between the two groups was not significant (P=0.098). As shown in Figure 2B, 7 of 29 low-score patients (24.1%) and 6 of 12 high-score patients (50.0%) had remnant lymph node after CCRT; this difference was also not significant (P=0.146).
Factors affecting DFS
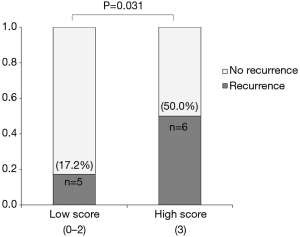
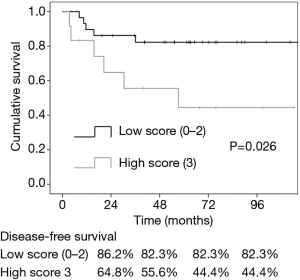
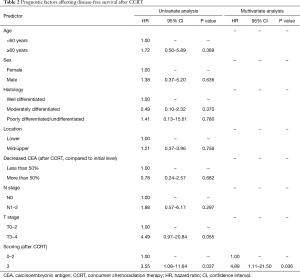
During the follow-up period, 11 patients experienced recurrence: four patients were identified as having local recurrence at the anastomosis site, and seven patients were found to have distant metastasis to the liver, lung, bone, or distant lymph nodes. Five patients with low-score (17.2%) and 6 patients with high-score (50.0%) experienced recurrence during follow-up (Figure 3); this difference was statistically significant (P=0.031). Figure 4 shows the Kaplan-Meier curves for cumulative DFS values according to scoring. Cumulative DFS value was 86.2% for low-score patients and 64.8% for high-score patients 24 months after diagnosis, and cumulative DFS value was 82.3% for low-score patients and 55.6% for high-score patients 48 months after diagnosis. In univariate Cox proportional-hazard regression models, a high score was the only significant factor affecting poor DFS [high score vs. low score: hazard ratio (HR) =3.55, 95% confidence interval (CI): 1.08–11.64, P=0.037]. In our multivariate model, high score was an independent risk factor for poor DFS (high score vs. low score: HR =4.89, 95% CI: 1.11–21.50, P=0.036; Table 2).
Full table
Discussion
In this study, we evaluated the clinical significance of endoscopic findings after CCRT in patients with rectal cancer using a simple scoring system. When analyzed long-term outcomes according to endoscopic scoring which divided into two groups of low score (≤2) and high score (3), we found that a high post-CCRT endoscopic score was a significant predictor of poor long-term outcome. According to our results, simple post-CCRT endoscopic scoring could be helpful to predict prognosis in patients with rectal cancer.
Neoadjuvant CCRT is the standard treatment for patients with locally advanced rectal cancer (18). Patients who received CCRT also received subsequent surgery and additional chemotherapy. Neoadjuvant CCRT has proven effective at reducing local recurrence and is associated with increased rates of sphincter preservation (4,5). Recent studies showed that neoadjuvant CCRT could lead to reduced tumor volume and decreased lymph node yield (19,20). Herein, we evaluated simple endoscopic scoring to predict T and N staging after CCRT. The T3–4/T0–2 and N1–2/N0 ratios were higher in the high-score patients compared with the low-score patients. The ratio differences between the score groups were not statistically significant, and scoring was not a significant factor affecting T or N stage after CCRT (data now shown).
Previous studies have suggested that the response to neoadjuvant CCRT, including tumor regression grade as assessed by magnetic resonance imaging or pathology, was correlated with long-term outcomes in patients with rectal cancer (12,14,21). Herein, we aimed to evaluate tumor regression grade according to endoscopic findings and to assess the association between endoscopic findings and long-term outcomes in patients with rectal cancer. In an analysis of recurrence rate according to endoscopy score, patients with low score had significantly lower recurrence during follow-up than patients with high score (17.2% vs. 50.0%, P=0.031). According to Kaplan-Meier curves, DFS for low-score patients was significantly longer than for high-score patients (P=0.026). In multivariate Cox-hazard regression analysis, a high score was an independent risk factor for poor DFS (HR =4.89, 95% CI: 1.11–21.50, P=0.036). These results suggest that assessment of post-CCRT endoscopic findings can provide useful insights into long-term outcomes in patients with rectal cancer.
The Mayo endoscopic score is the most commonly used score for ulcerative colitis, and it involves four categories (score: 0–3) that comprise a disease activity index for ulcerative colitis patients (22). In this study, we aimed to create a simple scoring system similar to the Mayo endoscopic score, and we classified post-CCRT endoscopic findings of patients with rectal cancer into four categories (score: 0–3), as shown in Figure 1. However, endoscopic assessment is limited in that it involves the subjectivity of endoscopists. To minimize errors due to this limitation, two experienced gastroenterologists reviewed all endoscopic findings several times and determined endoscopic scores through agreement. Endoscopic findings categorized as high scores (3) are provided in Figure S1.
This study has several limitations. First, this is a retrospective study performed in a single center, and we could not avoid selection bias in our patient sample. However, we tried to minimize any biases by repeatedly reviewing all medical records. Second, the number of patients in this study is small, which could reduce the reliability of our results. Third, as mentioned above, endoscopic image assessment depends on endoscopist subjectivity. To overcome these limitations, further prospective studies with larger patient groups are needed. Additionally, the simple endoscopic scoring system used in this study needs further validation.
In conclusion, our study showed that a high score in a simple post-CCRT endoscopic scoring system is a useful factor to predict poor long-term outcomes in patients with rectal cancer. Based on these study results, we suggest that assessment of post-CCRT endoscopic findings in patients with rectal cancer is important for predicting long-term outcomes.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Institutional Review Board of Kosin University Gospel Hospital (KUGH 2019-04-006).
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- Cancer Today: data visualization tools for exploring the global cancer burden in 2018. [Internet]. Lyon: International Association of Cancer Registries; c2018 (cited 2019 Mar 28). Available online: https://gco.iarc.fr/today/home
- Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet 2014;383:1490-502. [Crossref] [PubMed]
- Wagman R, Minsky BD, Cohen AM, et al. Sphincter preservation in rectal cancer with preoperative radiation therapy and coloanal anastomosis: long term follow-up. Int J Radiat Oncol Biol Phys 1998;42:51-7. [Crossref] [PubMed]
- Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731-40. [Crossref] [PubMed]
- Maas M, Nelemans PJ, Valentini V, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol 2010;11:835-44. [Crossref] [PubMed]
- McCarthy K, Pearson K, Fulton R, et al. Pre-operative chemoradiation for non-metastatic locally advanced rectal cancer. Cochrane Database Syst Rev 2012;12:CD008368. [PubMed]
- Ceelen WP, Van Nieuwenhove Y, Fierens K. Preoperative chemoradiation versus radiation alone for stage II and III resectable rectal cancer. Cochrane Database Syst Rev 2009.CD006041. [PubMed]
- De Caluwé L, Van Nieuwenhove Y, Ceelen WP. Preoperative chemoradiation versus radiation alone for stage II and III resectable rectal cancer. Cochrane Database Syst Rev 2013.CD006041. [PubMed]
- Rahbari NN, Elbers H, Askoxylakis V, et al. Neoadjuvant radiotherapy for rectal cancer: meta-analysis of randomized controlled trials. Ann Surg Oncol 2013;20:4169-82. [Crossref] [PubMed]
- Peeters KC, van de Velde CJ, Leer JW, et al. Late side effects of short-course preoperative radiotherapy combined with total mesorectal excision for rectal cancer: increased bowel dysfunction in irradiated patients--a Dutch colorectal cancer group study. J Clin Oncol 2005;23:6199-206. [Crossref] [PubMed]
- Fokas E, Liersch T, Fietkau R, et al. Tumor regression grading after preoperative chemoradiotherapy for locally advanced rectal carcinoma revisited: updated results of the CAO/ARO/AIO-94 trial. J Clin Oncol 2014;32:1554-62. [Crossref] [PubMed]
- Fokas E, Ströbel P, Fietkau R, et al. Tumor Regression Grading After Preoperative Chemoradiotherapy as a Prognostic Factor and Individual-Level Surrogate for Disease-Free Survival in Rectal Cancer. J Natl Cancer Inst 2017. [Crossref] [PubMed]
- Park IJ, You YN, Agarwal A, et al. Neoadjuvant treatment response as an early response indicator for patients with rectal cancer. J Clin Oncol 2012;30:1770-6. [Crossref] [PubMed]
- Collette L, Bosset JF, den Dulk M, et al. Patients with curative resection of cT3-4 rectal cancer after preoperative radiotherapy or radiochemotherapy: does anybody benefit from adjuvant fluorouracil-based chemotherapy? A trial of the European Organisation for Research and Treatment of Cancer Radiation Oncology Group. J Clin Oncol 2007;25:4379-86. [Crossref] [PubMed]
- Al-Sukhni E, Attwood K, Mattson DM, et al. Predictors of Pathologic Complete Response Following Neoadjuvant Chemoradiotherapy for Rectal Cancer. Ann Surg Oncol 2016;23:1177-86. [Crossref] [PubMed]
- Sohn DK, Han KS, Kim BC, et al. Endoscopic assessment of tumor regression after preoperative chemoradiotherapy as a prognostic marker in locally advanced rectal cancer. Surg Oncol 2017;26:453-9. [Crossref] [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practive Guidelines in Oncology. Colon Cancer ver 1.2019. Available online: https://www.nccn.org/professionals/physician_gls/default.aspx#colon
- Li Y, Wang J, Ma X, et al. A Review of Neoadjuvant Chemoradiotherapy for Locally Advanced Rectal Cancer. Int J Biol Sci 2016;12:1022-31. [Crossref] [PubMed]
- Miller ED, Robb BW, Cummings OW, et al. The effects of preoperative chemoradiotherapy on lymph node sampling in rectal cancer. Dis Colon Rectum 2012;55:1002-7. [Crossref] [PubMed]
- Patel UB, Taylor F, Blomqvist L, et al. Magnetic resonance imaging-detected tumor response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience. J Clin Oncol 2011;29:3753-60. [Crossref] [PubMed]
- Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med 1987;317:1625-9. [Crossref] [PubMed]