Accuracy of nodal staging is influenced by sidedness in colon cancer
Introduction
Although surgical resection is the most effective treatment for patients with colon cancer (CC), a considerable number of patients will develop recurrence. Therefore, adjuvant chemotherapy (AC) plays an important role in its management (1). The decision to consider AC is largely determined by lymph node (LN) status. Accurate nodal evaluation is essential for accurately staging patients and making decisions regarding AC (2). Current guidelines define adequate lymph node (LN) sampling, the number of nodes needed to accurate stage patient with CC, as ≥12 LNs (3,4). This recommendation was largely based on studies that reported adequate true nodal negativity (TNN) and low false negative rate when 12 or more LNs were examined (5,6).
It is recommended that patients with LN-positive disease and those with T4 disease regardless of their LN status receive AC (3,4). The decision to administer AC in patients with node-negative T3 continues to be a subject of much debate. The National Cancer Care Network (NCCN) recommends considering AC in patients with T3N0 with high-risk features that put them at high risk for recurrence or when less than 12 LNs are examined (3). This recommendation is based on the notion that examining fewer than 12 LNs may not reliably predict TNN and hence, the decision to not consider AC may not be correct. Thus, accurately predicting true nodal negative status in this subset of patients is critical.
Tumor sidedness impacts clinical presentation, response to surgical and non-surgical therapy, as well as prognosis after treatment (7,8). There is a paucity of data regarding the impact of tumor sidedness on nodal staging accuracy. Therefore, we sought to examine the impact of tumor sidedness on number of LNs needed to accurately stage patients with T3N0 CC, as the decision to administer chemotherapy in this subgroup of patients is largely based on the number of examined LNs.
Methods
Study design and cohort selection
A retrospective study was conducted on a cohort of patients with pathologic T3 CC with at least one LN examined who were identified from a prospective, multicenter, international trial of ultrastaging in CC (ClinicalTrials.gov ID: NCT00949312). Information on age, gender, race/ethnicity, N stage, grade, number of harvested nodes, and chemotherapy was obtained. The probability of true nodal negativity (TNN), defined as the probability of accurately determining the negative nodal status, was calculated for right CC (RCC) and left CC (LCC). A mathematical model, using a method previously used (6), was used to estimate the probability of TNN.
A cohort of patients with similar inclusion criteria was identified from the National Cancer Database (NCDB) and formed the validation cohort of this study. The NCDB is a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society. Established in 1989 the NCDB is a nationwide, facility-based, comprehensive clinical surveillance resource oncology data set that currently captures 70% of all newly diagnosed malignancies in the US annually (9).
Patients aged ≥18 years with pathologic T3, primary CC, and had at least one LN removed at the time of surgery were included. Similarly, data on age, gender, and race/ethnicity, N stage, grade, number of harvested nodes, and chemotherapy was obtained. For the purposes of this study, tumors were classified as “right colon” if they were found in the cecum, ascending colon, or transverse colon, and “left colon” if they were in the splenic flexure, descending or sigmoid colon.
Statistical analysis
Baseline characteristics by tumor sidedness were compared using Chi-squared and Fisher’s exact tests for categorical and continuous variables, respectively. To estimate the probability of TNN based on the number of LNs examined, we adopted the Bayesian model (10) based on two quantities:
- The probability of having M positive nodes, P(M), was empirically estimated by P(M) = (number of patients with M positive nodes)/(total number of patients in group).
- The probability of m positive nodes observed in a sample of n nodes examined over a total of N nodes, among which M nodes are positive, P(m|M;N,n), is obtained from a hypergeometric distribution as follows:
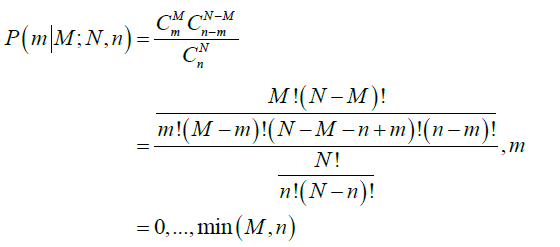
The average total number of lymph nodes was assumed to be 44 in the right colon and 46 in the left colon (6). These numbers were obtained from the average number of lymph nodes seen in previous studies (11). The probability that a patient truly has M positive nodes after m nodes were observed positive in a sample of n nodes examined is obtained by Bayesian theorem:
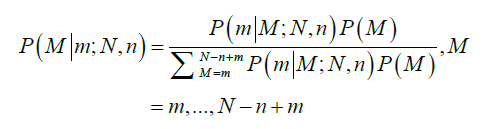
Therefore, the probability of TNN based on the number of LNs examined, n, is estimated as follows:
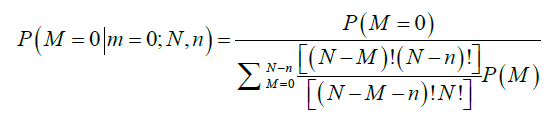
The probability of TNN was calculated for both RCC and LCC based on the number of examined nodes for the trial and the NCDB cohort. A series of Kaplan-Meier survival analyses were then performed for both RCC and LCC according to different cutoffs for the number of nodes examined. However, this was only done for the NCDB but not the trial cohort due to sample size limitation. Hazard ratios were generated using univariate Cox proportional regression analysis. First analysis was based on the current threshold of 12 LNs (1–11 vs. ≥12). Similar analyses were then performed based on the number of LNs needed to achieve three different cutoffs for the probability of TNN (85%, 90%, and 95%) for both RCC and LCC. All statistical analysis was performed using R software (R Foundation for Statistical Computing, Vienna, Austria).
Results
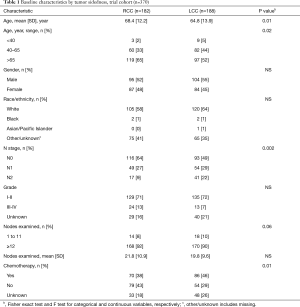
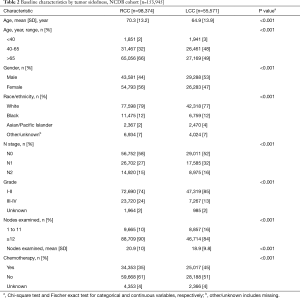
Three hundred and seventy patients met the inclusion criteria in the trial cohort; 49% were RCC and 48% were LN-negative. Of 153,945 patients in the NCDB, 57% were LN-negative and 64% were RCC. In both cohorts, patients with LCC were younger and more likely to have node-positive disease. The mean number of examined nodes and the proportion of patients with ≥12 LNs were higher in RCC compared to LCC in both cohorts. Chemotherapy was given more frequently to patients with LCC than RCC in both Cohorts (Tables 1,2).
Full table
Full table
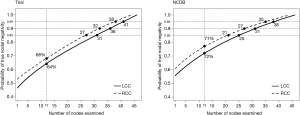
The probability of TNN increased as the number of examined nodes increased in both cohorts. The probability of TNN when 12 LNs were examined was 68% for RCC and 64% for LCC in the trial cohort and 77% and 72% in the NCDB (Figure 1). The number of nodes needed to achieve any given probability of TNN was significantly different between RCC and LCC in both the trial (P<0.001) and the NCDB (P<0.001). More LNs are needed to achieve the same probability of TNN in patients with LCC than RCC. The number of nodes needed to achieve an 85% probability of TNN was 27 for RCC vs. 31 for LCC and 21 vs. 25 in the trial and NCDB cohort; respectively. To achieve a 90% probability of TNN, 32 LNs needed to be examined for RCC versus 36 for LCC in the trial cohort and 27 vs. 31 in the NCDB cohort. To reach a 95% probability, those numbers were 38 vs. 41 and 35 vs. 38 in the trial and NCDB cohort; respectively (Figure 1).
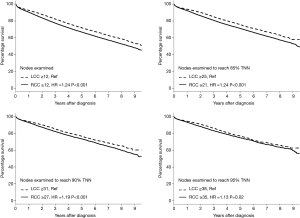
Figure 2 illustrates four Kaplan-Meier survival analyses based on different cutoff points for the number of examined LNs using the NCBD. The first analysis was based on the current threshold of 12 LNs (1–11 vs. ≥12) stratified by sidedness. Compared to LCC ≥12 LNs as the reference group, patients with RCC ≥12 LNs had worse survival (HR 1.24; P<0.001). Further analyses were then performed based on the number of LNs needed to achieve three different cutoffs for the probability of TNN (85%, 90%, and 95%) for both RCC and LCC. When higher numbers of LNs were examined, survival improved for both RCC and LCC. Interestingly however, with higher numbers of LNs being examined, the magnitude of improvement in survival was higher for RCC than LCC and the difference in survival between RCC and LCC became smaller as the probability of TNN increased (HR for RCC vs. LCC =1.24 with 85% TNN, 1.19 with 90% TNN, and 1.13 with 95% TNN).
Discussion
We demonstrate that tumor sidedness influences the number of nodes that need to be examined to say with high probability that a patient is truly node negative. This is particularly relevant to patients with T3N0 disease. First, we showed that examining 12 LNs does not reliably yield a TNN on either side. Second, we demonstrated that sidedness influences the accuracy of nodal staging in both a prospective multicenter trial and NCDB. The number of LNs needed to accurately predict any given probability of TNN was significantly higher in LCC than RCC. Furthermore, as the probability of TNN improves, survival improves for both RCC and LCC. The magnitude of improvement in survival with more LNs examined, however, is greater for RCC than LCC.
A secondary analysis of patients from the intergroup trial INT-0089 showed that an increase in the number of LNs examined was associated with improved survival for patients with both node-negative and node-positive disease (12). In addition, results from population-based studies show an association between an improvement in survival and examination of greater than or equal to 12 LNs (6,13). The reasons for this are not well understood although stage migration is one hypothesis—harvesting more LNs is more likely to identify positive LN’s and therefore select patients for AC (14,15). Some studies have suggested that other factors associated with LN harvest such as the extent and quality of surgical resection may have an impact on survival (16). Another hypothesis is that the immune system and the underlying tumor biology may affect both tumor biology and prognosis (17).
More than half of patients with CC are pathologically categorized as having T3 disease. According to the AJCC, T3 is defined as transmurally invasive tumors that are confined to the perimuscular soft tissue (i.e., that have neither violated the serosal surface nor infiltrated an adjacent structure) (18). Studies have shown that nodal metastases in patients with T3 tumors is contingent upon the number of examined LNs. Ong et al. recently reported that nodal metastasis were found in 22% of specimens with fewer than 15 LNs compared to 85% with 15 or greater recovered nodes (19). Although national and international guidelines recurrently recommend that at least 12 LNs should be examined to ensure adequate staging of CC, several studies have suggested different cutoffs for LN harvest for T3 patients to ensure more accurate nodal staging (20).
The NCCN recommends examination of a minimum of 12 LNs to accurately stage CC (3). This recommendation is supported by the College of American Pathologist (21) and the American Joint Committee on Cancer (18), and it has been adopted by the Commission on Cancer (22) as a quality of care measure. For resected stage II (pT3N0) CC, if fewer than 12 LNs are harvested, it is recommended that the pathologist go back to the specimen and resubmit more tissue for more potential LNs (3). NCCN guidelines suggest that patients with less than 12 negative LN’s may be sub-optimally staged and should be considered to be at high risker risk for recurrence.
Recent studies have demonstrated that RCC and LCC have different epidemiological, biological, clinical characteristics, and outcomes (7,23). This raises the question whether a different threshold for the number of examined nodes should be applied to different segments of the colon, particularly in patients with T3 disease. The prevalence of nodal positivity due to false- negatives is observed for all T stages, but its extent increases with T stage. In T3 disease, the false-negative rate has been estimated at 57%, 39%, 18% and less than 10% for 1, 3, 10 and greater than 20 nodes examined, respectively (24). In this study, we estimated the probability of TNN as a function of both the number of examined LNs and tumor sidedness. This may provide a more accurate guide rather than a simple cutoff of the number of examined nodes, and therefore improve the selection of patients for AC.
One interesting finding in our study was the decrease in the survival gap between RCC and LCC with the increase in the number of examined nodes. This confirms the findings from a recent study of 504,958 patients with stage I-III CC which reported improved survival in patients with RCC with a higher LN harvest, ≥22 nodes had the highest OS (HR 0.71, 95% CI: 0.68–0.75) (25). Previous studies have proposed that the lack of routine high ligation of the ileocolic pedicle in RCC, as compared to IMA high ligation for LCC, may result in different nodal harvest patterns between the RCC and LCC and could explain, in part, the worse survival with the former (26). However, in our study, the mean number of examined nodes were higher in RCC compared to LCC in both cohort which is consistent with other studies (25,27).
Given the retrospective nature and a few key assumptions required for the calculation of TNN, there are several limitations to this study. First, the average total number of LNs was assumed to be 44 in the right colon and 46 in the left colon. These numbers were used in previous landmark studies (6,11). Second, the “Ultra-staging in CC” trial enrolled patients from medical centers located within the United States, Serbia and Israel, whereas the NCDB is a nationwide comprehensive clinical surveillance oncology data set. This may explain the differences in some of the baseline characteristics as well as the probability of TNN.
The NCDB is a large national database and has limitations related to its retrospective design and reliance on accurate coding and data entry. Lymphovascular invasion was insufficiently captured in the NCDB, therefore it was not included in the final analysis. Additionally, there is variation in surgical technique and lack of standardization for pathologic analysis. These may have influenced the accuracy of the reported number of LNs, especially in the NCDB. The aim of this study however was to examine the hypothesis of whether sidedness should be factored in when determining the minimum number of LNs needed to accurately stage T3N0 CC and this was not impacted by the limitations of the NCDB cohort.
Conclusions
Current guidelines regarding the minimum number of LNs needed to accurately stage patients with T3N0 CC may need to consider tumor sidedness as an important factor particularly as it relates to the selection of patients for AC. Future studies should focus on determining the number of LNs need to be examined on each side to ensure nodal staging accuracy and more informed AC decision making.
Acknowledgments
Funded by the California Oncology Research Institute.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Sharif S, O’Connell MJ, Yothers G, et al. FOLFOX and FLOX regimens for the adjuvant treatment of resected stage II and III colon cancer. Cancer Invest 2008;26:956-63. [Crossref] [PubMed]
- Wilkinson NW, Yothers G, Lopa S, et al. Long-term survival results of surgery alone versus surgery plus 5-fluorouracil and leucovorin for stage ii and stage iii colon cancer: Pooled analysis of NSABP C-01 through C-05. A baseline from which to compare modern adjuvant trials. Ann Surg Oncol 2010;17:959-66. [Crossref] [PubMed]
- Benson AB, Venook AP, Cederquist L, et al. Colon Cancer, Version 1.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017;15:370-98. [Crossref] [PubMed]
- Schmoll HJ, Van Cutsem E, Stein A, et al. Esmo consensus guidelines for management of patients with colon and rectal cancer. A personalized approach to clinical decision making. Ann Oncol 2012;23:2479-516. [Crossref] [PubMed]
- Goldstein NS. Lymph node recoveries from 2427 pT3 colorectal resection specimens spanning 45 years: Recommendations for a minimum number of recovered lymph nodes based on predictive probabilities. Am J Surg Pathol 2002;26:179-89. [Crossref] [PubMed]
- Joseph NE, Sigurdson ER, Hanlon AL, et al. Accuracy of determining nodal negativity in colorectal cancer on the basis of the number of nodes retrieved on resection. Ann Surg Oncol 2003;10:213-8. [Crossref] [PubMed]
- Benedix F, Kube R, Meyer F, et al. Comparison of 17,641 patients with right- and left-sided colon cancer: Differences in epidemiology, perioperative course, histology, and survival. Dis Colon Rectum 2010;53:57-64. [Crossref] [PubMed]
- Petrelli F, Tomasello G, Borgonovo K, et al. Prognostic Survival Associated With Left-Sided vs. Right-Sided Colon Cancer. JAMA Oncol 2017;3:211-9. [Crossref] [PubMed]
- American College of Surgeons, American Cancer Society. National Cancer Database. National Cancer Database. Available online: https://www.facs.org/quality-programs/cancer/ncdb
- Kiricuta CI, Tausch J. A mathematical model of axillary lymph node involvement based on 1446 complete axillary dissections in patients with breast carcinoma. Cancer 1992;69:2496-501. [Crossref] [PubMed]
- Herrera-Ornelas L, Justiniano J, Castillo N, et al. Metastases in Small Lymph Nodes From Colon Cancer. Arch Surg 1987;122:1253-6. [Crossref] [PubMed]
- Le Voyer TE, Sigurdson ER, Hanlon AL, et al. Colon cancer survival is associated with increasing number of lymph nodes analyzed: A secondary survey of intergroup trial INT-0089. J Clin Oncol 2003;21:2912-9. [Crossref] [PubMed]
- Swanson RS, Compton CC, Stewart AK, et al. The prognosis of T3N0 colon cancer is dependent on the number of lymph nodes examined. Ann Surg Oncol 2003;10:65-71. [Crossref] [PubMed]
- Wong JH, Severino R, Honnebier MB, et al. Number of nodes examined and staging accuracy in colorectal carcinoma. J Clin Oncol 1999;17:2896-900. [Crossref] [PubMed]
- Wong SL, Ji H, Hollenbeck BK, et al. Hospital lymph node examination rates and survival after resection for colon cancer. JAMA 2007;298:2149-54. [Crossref] [PubMed]
- Wong SL. Lymph node evaluation in colon cancer: Assessing the link between quality indicators and quality. JAMA 2011;306:1139-41. [Crossref] [PubMed]
- Pagès F, Berger A, Camus M, et al. Effector Memory T Cells, Early Metastasis, and Survival in Colorectal Cancer. N Engl J Med 2005;353:2654-66. [Crossref] [PubMed]
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th Edition of the AJCC Cancer Staging Manual and the Future of TNM. Ann Surg Oncol 2010;17:1471-4.
- Ong MLH, Schofield JB. Assessment of lymph node involvement in colorectal cancer. World J Gastrointest Surg 2016;8:179-92. [Crossref] [PubMed]
- Gönen M, Schrag DM, Weiser MR. Nodal staging score: A tool to assess adequate staging of node-negative colon cancer. J Clin Oncol 2009;27:6166-71. [Crossref] [PubMed]
- Bostwick DG, Grignon DJ, Hammond MEH, et al. Prognostic factors in prostate cancer: College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med 2000;124:995-1000. [PubMed]
- Definitions M, Type M, Improvement Q. Cancer Programs Practice Profile Reports (CP 3 R) Rapid Quality Reporting System (RQRS) 2013;3:1-12. Available online: https://www.facs.org/quality-programs/cancer/ncdb/qualitytools/rqrs
- Kim SR, Song N, Yothers G, et al. Tumour sidedness and intrinsic subtypes in patients with stage II/III colon cancer: Analysis of NSABP C-07 (NRG Oncology). Br J Cancer 2018;118:629-33. [Crossref] [PubMed]
- Wu Z, Qin G, Zhao N, et al. Assessing the adequacy of lymph node yield for different tumor stages of colon cancer by nodal staging scores. BMC Cancer 2017;17:498. [Crossref] [PubMed]
- Lee L, Erkan A, Alhassan N, et al. Lower survival after right-sided versus left-sided colon cancers: Is an extended lymphadenectomy the answer? Surg Oncol 2018;27:449-55. [Crossref] [PubMed]
- Bertelsen CA, Kirkegaard-Klitbo A, Nielsen M, et al. Pattern of Colon Cancer Lymph Node Metastases in Patients Undergoing Central Mesocolic Lymph Node Excision: A Systematic Review. Dis Colon Rectum 2016;59:1209-21. [Crossref] [PubMed]
- Yang L, Xiong Z, Xie Q, et al. Prognostic value of total number of lymph nodes retrieved differs between left-sided colon cancer and right-sided colon cancer in stage III patients with colon cancer. BMC Cancer 2018;18:558. [Crossref] [PubMed]