Techniques of hepatic resection
History
Liver surgery has been described for centuries in literature in relationship to patients being treated for stab wounds and other injuries to the liver. However it wasn’t until development in general anesthesia and antibiotics that formal liver resections became more prevalent. In 1886, Dr. Luis performed the first liver surgery but the patient died 6 hours later due to bleeding. The first successful liver resection is attributed to Dr. Langenbuch in 1888, although the patient was reoperated on for bleeding (1). These complications did not stop surgeons from studying the anatomy to find successful ways to resect liver tumors. In 1897, Dr. Cantlie’s description of the liver established further understanding of liver anatomy, leading to better control of bleeding during surgery. Kousnetzoff and Pensky in 1896 described the suture fracture technique that allows compression and ligation of vasculature while transecting the parenchyma, a technique that is still used today with some variations (1). One of the seminal contributions to liver resections came from Dr. Pringle, who in 1908 described the technique of compressing portal inflow to decrease liver bleeding. Further improvement in operative exposure using subcostal incisions with retraction has also lead to a decrease in operative morbidity and mortality (1). In the last 60 years, technological advances have led to a rapid development in various techniques in liver resection. In this review article, we discuss specifically techniques in liver resection for metastatic colorectal cancer to the liver. We find that there is not just one specific way that works the best. Tumors in different lobes of the liver will require a different approach with the specific technique tailored to each patient.
Anatomy
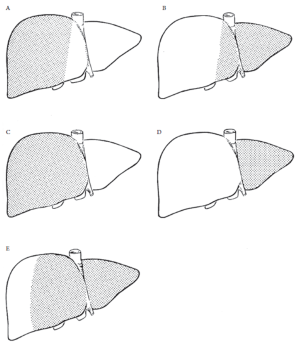
Surgery of the liver is based largely on the anatomic description of functional segments, which in turn is based on the organ’s blood supply via the hepatic artery and portal vein, its venous drainage via the hepatic veins, and finally, its biliary drainage. This division of the liver into eight functional segments is the most widely-accepted anatomic definition used in the context of hepatic resections (2-4). Major hepatic resections may be safely accomplished by adequately comprehending this internal segmental anatomy and its relationship to the major vascular structures (Figure 1).
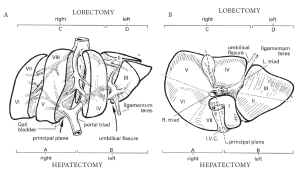
The anatomic right and left lobes of the liver are divided by the ligamentum teres and umbilical fissure, where the main vascular and biliary structures to the functional left liver run. However, the true functional division of the right and left liver is divided by the middle hepatic vein. This can be demarcated by a plane extending from the left side of the gallbladder fossa anteriorly, to the left side of the inferior vena cava posteriorly (known as Cantlie’s line). The right and left liver are further subdivided into segments which follow the distribution of the portal triad structures. The right, middle, and left hepatic veins drain into the vena cava and run within the corresponding scissurae.
The left liver is divided by the falciform ligament into a medial and lateral segment. The left lateral segment is divided into a superior (segment II) and inferior segment (segment III) by the left portal vein. The left medial segment (segment IV) is also divided into a superior portion (IVa) and an inferior portion (IVb). These divisions correlate to branches from the portal vein. The right liver is divided into an anterior (segments 5, 8) and posterior segments (segments 6, 7) by the right hepatic vein. These segments are further subdivided into inferior and superior segments by the right portal vein. Thus, there are four segments that comprise the right liver: anteroinferior (medial, segment V), posteroinferior (lateral, segment VI), posterosuperior (lateral, segment VII), and anterosuperior (medial, segment VIII). The caudate lobe (segment I) is posterior and inferior in relationship to the rest of the liver, and lies over the inferior vena cava. It receives portal irrigation from both right and left branches and drains directly into the vena cava.
The terminology of major hepatic resections arises from the segmental anatomic description above (Figures 1, 2). Right hepatectomy (or hemihepatectomy) involves resection of segments V-VIII, whereas left hepatectomy involves resection of segments (II-IV). Right lobectomy (also known as extended right hepatectomy, or right trisegmentectomy) involves resection of all segments lateral to the umbilical fissure (IV-VIII, and sometimes I), whereas extended left hepatectomy (or left trisegmentectomy includes resection of all liver medial to the umbilical fissure and a portion of the right liver (segments II-IV and segments V and VIII). Left lobectomy (also known as left lateral segmentectomy) involves resection of all liver medial to the umbilical fissure only (segments II and III) (1,5).
Types of Major Resections
An important decision in any liver resection is choosing the amount of parenchyma to be removed. Anatomic resections usually involve 2 or more hepatic segments, while non-anatomic resection involves resection of the metastases with a margin of uninvolved tissue (segmentectomy). This decision regarding extent of resection becomes especially relevant in the setting of post preoperative chemotherapy in colorectal metastasis, where an attempt to maximize the remnant liver volume is made. While preoperative therapy allows more patients to be considered resectable, it can compromise hepatic function and increase the risk of postoperative liver failure (6). Thus, the choice to perform a non-anatomic, or wedge resection should consider key factors such as preoperative chemotherapy, pre-existing liver disease, tumor burden, risk of recurrence, and whether or not outcome will be affected by the extent of resection (7). The greater parenchymal-sparing surgery afforded by a non-anatomical resection may prove to be beneficial especially in the setting of intrahepatic recurrent disease, which occurs in up to 50% of cases, where local minimally-invasive ablative therapies may be more amenable.
A small series of patients who underwent initial partial hepatic resection and recurred thereafter was reported by van der Pool and colleagues. They demonstrated that repeat treatment for recurrence of intrahepatic disease with local therapies (which included repeat non-anatomic resection, radiofrequency ablation, or stereotactic radiation) can be performed safely and with good median overall survival (37 months) and an overall 5-year survival rate of 35% in their series (8). A recent Dutch retrospective study compared the difference in morbidity and mortality and the patterns of recurrence and survival in 201 patients with colorectal liver metastases treated initially with anatomic versus nonanatomic liver resection. The trial found that nonanatomic resection was typically performed for significant smaller metastases, and this group received significantly less blood transfusions and had a shorter hospital stay. The groups did not differ with regards to morbidity, mortality, recurrence rate, or survival according to resection type (9). In similar fashion, multiple previous studies comparing anatomic vs. non-anatomic resection for colorectal liver metastases have not demonstrated any significant differences with regards to survival, margin status, or patterns of recurrence (10-12).
Vascular Control
Blood loss is among the most important variables influencing postoperative outcome from hepatic resection (13). In order to perform liver resections safely and to minimize blood loss and need for blood transfusions, it is essential to be familiar with different hepatic vascular occlusion techniques available. The application of each individual technique should be based upon the type of resection to be performed, tumor size and location, and preoperative liver function. More importantly, the different methods of vascular control each have distinct physiologic and hemodynamic effects systemically and within the liver itself, and thus the choice of which method to use should be determined by the patient’s ability to tolerate it. The array of vascular occlusion techniques ranges from Pringle’s maneuver (portal triad clamping) to total hepatic vascular exclusion, including inflow occlusion (selective or total), hemi-hepatic clamping, and ischemic pre-conditioning. These methods can also vary with regards to timing and frequency (intermittent vs. continuous) (14).
Inflow occlusion by hepatic pedicle clamping has been shown to reduce blood loss during liver resection (15). This is a consistent method of vascular control, which is not technically very difficult to perform. While it addresses the portal vein and hepatic artery, it does not address backbleeding from the hepatic veins. The Pringle maneuver can be performed continuously or intermittently and is usually well tolerated by the liver. When performed intermittently, the portal triad is typically clamped for 10 minutes and then unclamped for 3 minutes (the clamping on and off can vary). This allows for a longer total occlusion time of up to 2 hours in the normal liver, which can be useful for more prolonged complex liver resections, as demonstrated in previous studies (16). The increased blood loss during the periods of unclamping can be a challenge; however, the total blood loss or transfusion requirements does not differ between the intermittent and continuous techniques (17).
A potential consequence of the intermittent technique is hepatocyte injury from a sequence of ischemia-reperfusion periods. However, a prospective, randomized study by Clavien, et al. demonstrated that a 10 minute sequence of ischemia and reperfusion preceding a longer 30 minute period of continuous vascular occlusion was a protective strategy in humans. In their study, these findings were more effective for younger patients requiring a prolonged period of inflow occlusion (18). This strategy of ischemic preconditioning when compared with intermittent Pringle did not show any significant difference in terms of blood loss in a recent meta-analysis (19).
The continuous Pringle maneuver allows for shorter total occlusion time, and has the advantage of avoiding interruption of the parenchymal transaction (20). Belghiti and colleagues nevertheless demonstrated that this does not necessarily translate into shorter overall operative time (21). Both the continuous and intermittent methods should be used for shorter time periods in the setting of chronically diseased livers or patients that have undergone preoperative chemotherapy. In the setting of chronic liver disease, the intermittent method has been shown to be better tolerated (22).
Total hepatic vascular exclusion is another method of reducing blood loss during liver resection by occluding the inflow and outflow. This technique mitigates the risk of retrograde hepatic vein bleeding and can decrease the risk of air embolism. Hepatic vascular exclusion is more technically difficult than pedicle clamping alone, as it requires complete mobilization of the liver and appropriate exposure of the inferior vena cava. This method may be performed by clamping the portal triad in addition to clamping the infrahepatic and suprahepatic vena cava, or more selectively by clamping the hepatic veins extraparenchymally and preserving caval flow. One of the major challenges of total hepatic vascular occlusion is the hemodynamic effects it induces, which may be poorly tolerated in up to 15% of patients (14). There is a 40-60% decrease in cardiac output and blood pressure, with the resulting compensatory mechanisms of tachycardia and increased systemic vascular resistance (23). It is associated with an increased risk of postoperative complications, increased operative time, and lacks significant benefit over portal triad clamping alone with regards to blood loss, transfusion requirements, and liver failure (14,24,25).
Another technique important in decreasing blood loss and operative time involves intrahepatic pedicle ligation. Ligation of the right, left or smaller branches of the portal vasculature supplying the portion of liver being resected is an important step in liver resection. The previous mentioned techniques of vascular control are important for controlling back bleeding from the adjacent segments of liver during transection. Understanding the anatomy of the portal vessels permits a safe approach to pedicle ligation. Portal triad consists of common hepatic duct, portal vein and hepatic artery (Figure 1). The triad is encased by Glisson’s capsule. As the portal triad enters the hilum of the liver, it splits into the right and left portal pedicles. The right further splits into the anterior and posterior branches. The left travels in the umbilical fissure and gives branches to segments II, III, IV. For right hepatectomy, a hepatotomy is made in segment IV in the gallbladder fossa and a second one made inferior to the main right portal branch near the caudate lobe. A blunt right angle or renal pedicle clamp is then passed superior from the hepatotomy in segment IV through liver parenchyma and then exiting via the inferior hepatotomy. A vascular clamp is used to compress this tissue and right portal pedicle allowing for demarcation of the right lobe. Once this is confirmed, a vascular stapler is used to transect the pedicle.
For a left hepatectomy, the hilar plate is elevated and the left portal pedicle is identified in the umbilical fissure. A hepatotomy is made at the level of lowering the hilar plate and a second hepatotomy in the back of segment II. The same clamp should be used to come around this pedicle with subsequent vascular clamping to check for demarcation and then a vascular stapler to transect the left portal pedicle. This technique used properly can decrease blood loss, decrease risk to injury of the hilum, and shortens operative time. Use of intraoperative US during pedicle ligation decreases injury to nearby vasculature. Pedicle ligation can be used in select cases needing segmentectomy. However, this maneuver should not be used for centrally located tumors because obtaining surgical margins will not be possible. For patients that cannot undergo pedicle ligation, the standard technique of isolating the hepatic artery, portal vein separately should be performed (extrahepatic ligation).
Parenchymal Transection
As the number of liver resections have increased over the past 20 years, so too has the armamentarium of surgical devices available to facilitate the different aspects of liver surgery such as vascular control, hemostasis, and parenchymal transection. This growing variety of tools has been especially represented in the field of parenchymal transection. The methods range from basic finger or clamp-fracturing the tissue, to devices based on more complex technology, such as ultrasonic or radiofrequency energy, water jet and tissue-sealing devices, and surgical staplers. These strategies are all aimed at reducing blood loss and transfusion requirements, and the increased postoperative complications associated with each. Additionally, there are other important factors to be considered when choosing a particular method, such as operative time, availability and ease of use, extent of hepatic injury affected, and cost. The use of one tool over the other will also vary according to the type of resection, and different techniques can be more advantageous in one setting than another. It is important to be familiar with many strategies and be able to apply them in the most appropriate setting. We discuss the most widely used methods at present and review the existing randomized data comparing them.
Crushing Technique
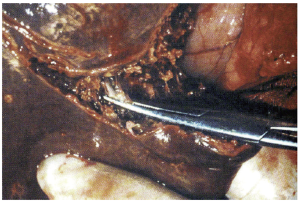
The most basic strategy involves crushing the liver parenchyma between the surgeon’s fingers in order to expose and isolate small vessels and biliary radicals, which can then be divided. This same fundamental technique can be further enhanced by employing basic surgical clamps (so-called “crush-clamp” technique) to crush the hepatic parenchyma, as shown (Figure 3). The crush-clamp method usually affords superior control when transecting the parenchyma as compared to the finger fracture method. Once the parenchyma is crushed, the exposed vessels and bile ducts can be divided. The latter can be achieved by silk suture ligation, bipolar electrocautery, vessel sealing devices, or vascular clips. Intermittent inflow occlusion with the Pringle maneuver is typically used during the transection and coagulation (Bovie cautery or argon beam coagulation) is applied to the remnant liver parenchyma during the periods of reperfusion for hemostasis. This technique is simple, quick, efficient, easy to learn and perform, and cost-effective. The crush-clamp strategy has served as the point of reference for all other hepatic parenchymal transection techniques. A series of randomized controlled trials and subsequent meta-analyses discussed below have analyzed and compared this method with newer ones.
A trial from Switzerland randomized 100 patients without cirrhosis or cholestasis to undergo liver resection using one of four methods: crush-clamp, ultrasonic dissector, water jet, or dissecting sealer (26). The patients randomized to the crush-clamp technique all underwent major hepatectomy with vascular inflow occlusion using a continuous Pringle maneuver, as opposed to the other groups in which routine Pringle maneuver was not used. The crush-clamp technique was associated with a shorter resection time, less blood loss, lower frequency of blood transfusion, and proved to be the most effective method. A subsequent German meta-analysis by Rahbari and colleagues analyzed seven randomized controlled trials with greater than 500 patients and found no clinically important benefit of an alternative transection method in terms of blood loss, parenchymal injury, transection time, and hospital stay (27). In similar fashion, a 2009 Cochrane review of randomized data failed to show any significant differences with regards to mortality, morbidity, markers of liver parenchymal injury, or ICU/hospital length of stay when comparing crush-clamp to alternative methods (28). The review did show crush-clamp to be faster and less expensive as well. Finally, the CRUNSH trial is a newly-designed prospective, randomized controlled trial comparing the efficacy of the crush-clamp technique versus use of a vascular stapler for parenchymal transection (29).
Ultrasonic Dissection
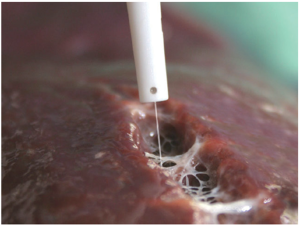
The Cavitron Ultrasonic Surgical Aspirator (CUSA, Tyco Healthcare, Mansfield, MA, USA) (Figure 4), combines ultrasonic energy with aspiration in order to divide the liver parenchyma and thus skeletonize small parenchymal vessels and biliary structures greater than 2mm, which are then divided according to preference. CUSA is able to dissect tissue but does not offer coagulation or hemostasis. Among CUSA’s benefits, it provides a very well-defined transection plane, which is useful in situations of close proximity between tumors and major vascular structures. Also, it can be used in cirrhotic as well as non-cirrhotic livers, and is associated with a low blood loss and low risk of bile leak (30). Transection time using CUSA is generally slower than conventional methods. Nonrandomized studies have shown decreased blood loss, morbidity, and mortality using CUSA, however, larger randomized trials have not shown this benefit over the traditional crush-clamp method (31).
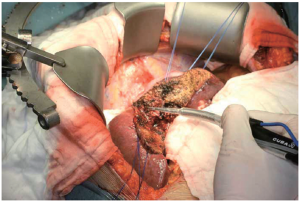
The Harmonic Scalpel (Ethicon Endo-Surgery, Cincinnati, OH, USA) uses a similar principle of ultrasound energy applied to vibrating ultrasonic shears to seal and divide blood vessels up to 3mm in diameter. The vibration of the blades at 55,500 times per second simultaneously cuts and coagulates tissues by causing denaturization of proteins, rather than heat, as with conventional electrocautery. This allows for a more precise transection plane and reduces lateral thermal damage as well. The Harmonic Scalpel finds its best application during laparoscopic liver resections. In a nonrandomized study by Kim, et al. use of the Harmonic Scalpel was associated with decreased operative time and a trend toward decreased blood loss and transfusion requirement. However, it was also associated with a significant increase in the incidence of postoperative bile leaks (32).
Sealing Devices
Sealing devices help in accomplishing liver parenchymal transection by sealing small vessels before division. These devices can be useful in the setting of laparoscopic or non-anatomic liver resections. The potential benefit of this technique involves simultaneous parenchymal division and vessel hemostasis, which can theoretically lead to decreased transection times. They are typically used in combination with other techniques or devices (33). The Ligasure Vessel Sealing System (Covidien, Mansfield, MA, USA) is a bipolar vessel sealing device which can permanently occlude blood vessels up to 7 mm in diameter by combining pressure and energy to fuse the collagen matrix in the vessel wall (Figure 5).
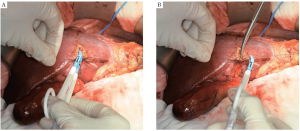
In a small single-center Japanese randomized trial comparing LigaSure to crush-clamp method, the former was associated with significantly lower blood loss, faster transection speed, and lower number of ties required (34). Postoperative bile leak was observed in three times more patients in the Ligasure group (3% vs. 9%), but this was not statistically significant. A more recent and larger randomized trial by Ikeda and colleagues, also from Japan, failed to show any significant decrease of the operation time or blood loss during liver transection as compared with that of the crush-clamp method (35).
Saline-Linked Radiofrequency Sealer
The Salient Dissecting Sealer (Salient Surgical Technologies, Portsmouth, NH, USA, formerly known as TissueLink) is a dissecting sealer that links radiofrequency energy with cool saline as a conductor at the cone-shaped tip of the device and thereby achieves blunt parenchymal dissection and hemostatic sealing of small vessels at the liver surface (Figure 6). Larger vessels are easily isolated and can be ligated and divided according to preference. The continuous saline irrigation cools the coagulated liver surface and prevents significant charring and eschar formation. This technique is also available for laparoscopic application. In one of the largest studies using this device for liver resection Geller, et al. achieved a considerably low rate of blood transfusions, bile leaks, and overall morbidity (36). This device is generally used for transecting cirrhotic livers. The radiofrequency sealer has also been used in the authors’ personal experience in obtaining adequate surgical margins when metastatic colorectal tumors are located near major biliary structures, due to its ablative nature. This provides an excellent additional tool in resecting tumors that would typically be considered unresectable due to their location near the major vascular and biliary structures; for example, central lesions localized at the bifurcation of the right and left portal structures.
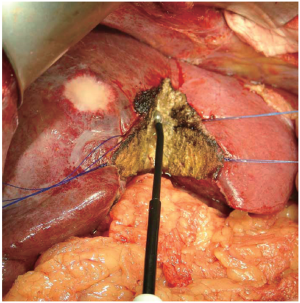
Radiofrequency-assisted Liver Resection
This technique applies radiofrequency energy to pre-thermocoagulate the liver parenchyma before division. The radiofrequency probe (Habib 4X, Angiodynamics, Queensbury, NY, USA) is used to treat the parenchyma along the plane of dissection for a few seconds, and thereby induces coagulative necrosis in a sphere of tissue around the probe (Figure 7). This leads to precoagulation of the tissue, which can then be transected with a scalpel (Figure 7). The radiofrequency energy is typically applied in sequentially overlapping segments to ensure adequate hemostasis. A randomized trial from Italy compared radiofrequency-assisted liver resection to crush-clamp and found a higher rate of postoperative complications (abscesses, biliary fistula, biliary stenosis) in the radiofrequency-assisted group (37). This technique causes a greater amount of tissue necrosis than the other techniques, which may lead to the infectious complications observed in their study. In addition, there is a theoretical potential for thermal insult to major biliary structures. Radiofrequency ablation is generally reserved for ablating liver tumors that are unresectable. However, it is not effective in tumors next to portal vessels due to the heat sink cause by blood flow. The liver parenchyma in these situations will not be high enough to cause the coagulation necrosis of the tumor.
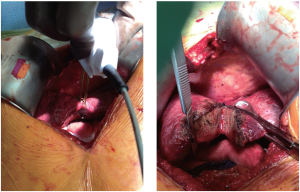
Water-jet Dissection
This technique employs a high-pressure water jet to break apart the liver tissue and selectively isolate small vascular and biliary structures, potentially decreasing blood loss. These vessels and ducts must then be ligated and divided individually according to preference. It is this necessary second step that may put this technique at a time disadvantage to others which offer simultaneous transection and hemostasis. Nonetheless, the precise delineation of the transection plane (Figure 8) produced by the water-jet dissector (ERBEJET 2, ERBE USA Inc, Marietta, GA, USA) can be advantageous for exposing the major vessels more effectively, especially in the context of closely adjacent tumors. Additionally, this technique spares the surrounding tissue from any thermal damage. Rau and colleagues presented a series of 350 liver resection performed exclusively with the water-jet dissector, in which they were able to reduce their blood loss, transfusion requirements, use of Pringle maneuver, and resection time in comparison to CUSA or blunt dissection (38). The data from randomized trials, however, has not shown a similar benefit (26,28,39).
Vascular stapler technique
Vascular staplers have become an accepted method of liver transection. Initially used primarily for division of major vessels, their use has been expanded to divide hepatic parenchyma. Staplers have the potential to be serially applied and fired in quick and efficient fashion, thus increasing their popularity. A common strategy is to use a large clamp to fracture the liver parenchyma along the line of transection, followed by serial firings of an Endo-surgical stapler with a vascular load (Ethicon Endo-Surgery, Cincinnati, OH, USA or Covidien, Mansfield, MA, USA) (Figure 9) (40,41). Reddy and colleagues published a retrospective series of greater than 200 patients over 10 years who underwent partial hepatectomy with either the crush-clamp alone or vascular stapler techniques. Compared to crush-clamp, use of a vascular stapler was associated with less operative time, blood loss, and transfusion requirements (42). The CRUNSH trial mentioned above, is a newly-designed prospective, randomized controlled trial comparing crush-clamp to vascular stapler in elective liver resections, and is currently recruiting participants (29).
Laparoscopic approach for liver resections
The role of laparoscopy in surgery is a growing field. Currently it is now utilized in liver resections in institutions experienced with minimally invasive surgical techniques. There are several different minimally invasive approaches ranging from total laparoscopic, hand assisted laparoscopic, to the more recent robotic assisted liver resections. There are about 3000 reported laparoscopic liver resections in the literature (43). The majority of cases have been done total laparoscopic followed by hand assisted laparoscopic. The most common liver resections performed laparoscopically are wedge resections, followed by left lateral segmentectomy (43,44). Generally, tumors in the periphery of the liver are also considered amenable to resection. Major hepatectomies (left or right hepatectomy) are not as commonly performed. In the series reviewed by Nguyen et al., only about 9% of cases were left or right hepatectomy. Conversion rate to open in the most experienced hands is reported at 4.1% (44). In 2008 a consensus meeting at the University of Louisville established guidelines for minimally invasive liver surgery (43). Indications for minimally invasive approach include solid tumors <5 cm, peripherally located tumors in segments 2-6, and major liver resections should be performed in highly experienced centers. The learning curve for minimally invasive laparoscopic liver resections currently remains at 60 cases. Data currently shows the benefit of minimally invasive technique to be decreased blood loss, shorter hospital stay, and decrease use of pain medication (44). In metastatic colorectal cancer, the reported negative margin is 94.4%, with overall survival of 50% at 5 years in patients (43). In experienced centers, there does not appear to be any difference in disease free or overall survival between open versus laparoscopic liver surgery. The technique involves using ultrasonic shears to dissect parenchyma with placement of clips on vessels or use of endo-GIA staplers for ligation of vasculature.
Use of the da Vinci robot (da Vinci Surgical System, Intuitive Surgical, Inc, Sunnyvale, CA, USA) has gained recent popularity amongst hepatobiliary surgeons in performing minimally invasive liver resections. It is widely accepted that a left lateral segmentectomy should be approached laparoscopically. Right and left hepatectomies are not often attempted due to the size of the liver resected, and difficulty with visualization and maneuvering of instruments. The robot attempts to eliminate these issues allowing surgeons to perform complex liver resections minimally invasive. The robot has the ability for 3D visualization, and wristed instruments which allow for more flexibility to perform fine movements not possible with laparoscopy. The largest series to date on robotic liver resections is from Italy and University of Chicago. Giulianotti and colleagues published a case series of 24 patients undergoing robotic right hepatectomy (45). Majority of the patients had metastatic colorectal cancer to the liver. Of the 24 patients, 1 was converted to open resection (tumor was adhesive to the inferior vena cava). Overall mean operative time was 337 minutes and mean blood loss 457cc. Only 3 patients required a blood transfusion. Mean hospital length of stay was 9 days. Parenchymal transection was done using harmonic shears and endo-staplers. There were no mortalities; however, there were a few complications such as bile leak (1 patient), transitory liver failure (2 patients), and a few other mentioned in the article. The results are very encouraging using the robot for minimally invasive approach. Long term survival data is not yet available. However, this approach should be performed in tertiary centers with experienced surgeons in minimally invasive as well as hepatobiliary surgery.
Loma Linda University approach for liver resections
At our institution we perform many complex liver resections. We have worked on standardizing an approach for liver resection that is safe, efficient, and cost-effective. We follow standard perioperative and postoperative management guidelines for patients. Patient positioning is supine with both arms out allowing anesthesia to access IV’s if necessary. A subcostal incision with a midline extension over the xiphoid allows for excellent exposure for the right lobe. A midline incision offers exposure for approaching the left lobe. The Thompson Retractor (Thompson Surgical Instruments, Traverse City, MI, USA) is our standard retractor used for exposure of the liver. It employs upward retraction of ribs that allow excellent exposure and ease in dissection of the suprahepatic IVC as well as infrahepatic IVC and mobilization of the right lobe of the liver.
Once exposure of the hepatic veins and mobilization of the liver is performed, intraoperative ultrasound is routinely used to identify the metastatic lesions, as well as looking for additional tumors not visualized on preoperative imaging. Intraoperative ultrasound also provides real time imaging of the liver allowing the surgeon to see the relationship of the tumor to vascular structures, assess resectability, and guide resection approach. In the case of metastatic colorectal cancer to the liver, intraoperative ultrasound also plays an important role in non-anatomic resection. With visualization and bimanual palpation of the liver, non-anatomic resections can be safely performed ensuring excellent surgical margins. The importance of intraoperative ultrasound was recently published by Hata et al. (46). They reviewed 65 hepatectomies retrospectively. Contrast enhanced liver CT scans had a sensitivity of 72.8% overall, but decreases to 34.6% for tumors less than 1cm. Using intraoperative ultrasound, detection of smaller tumors is possible and altered surgical resection in 72% of patients. Most alterations involved additional resection of liver parenchyma involved with tumor. The overall sensitivity of intraoperative ultrasound is about 98% (47). This data supports our routine use of intraoperative ultrasound allowing for a safe surgical approach.
Once an approach is determined for liver resection, the liver is mobilized and inflow and outflow vessels are controlled. Our approach for inflow control depends on the location of the tumor. For centrally located tumors that would require either a left hepatectomy or right hepatectomy, ligation of the portal vein and hepatic artery is done extrahepatic. For tumors located away from the bifurcation of the portal pedicles, intrahepatic pedicle ligation is performed. Next, outflow control of the hepatic veins is performed when either a right or left hepatectomy needs to be performed. The Pringle maneuver is applied intermittently, on for 5-10 minutes, and off for 2-3 minutes. We utilize the crush-clamp method for parenchymal dissection, along with the vascular stapler. Small vessels are clipped while larger ones are ligated with the stapler or suture ligature. Once the lobe or segment is removed, hemostasis is further achieved with argon beam coagulation. Avatine (Davol, Warwick, RI, USA) powder or other hemostatic agents are used along the surgical bed of the liver with packing for full hemostasis. Biliary leaks are controlled in the surgical bed with suture ligature. We do not routinely place surgical drains since this has not shown to improve outcome (48-50).
Conclusion
The continual success of liver surgery is owed to not only improved transection techniques, but also to advances in perioperative care, anesthesia, and post-operative care. From Kousnetzoff and Pensky description of suture fracturing method to surgeons sitting at a computer console using a robot to remove liver tumors, technological advances have been the single most entity that has revolutionized the safety of liver surgery. With more surgeons performing these procedures with the various mentioned techniques we have enough data to analyze if one technique is better than the other. The general approach in preparation of the patient and the liver is standardized in many institutions and surgical groups with regards to the patient position, instruments, and retractors. Most liver surgeons now use the Thompson Liver retractor which allows for ease of visualization of the hepatic veins and mobilization of the liver. Decisions about anatomic versus non-anatomic resections for colorectal metastasis should be made before surgical exploration. Preservation of as much liver parenchyma is important since many patients will receive preoperative chemotherapy and risks of liver failure are much higher. It is accepted that no more than six cycles of chemotherapy is to be used preoperatively. In situations where more than six cycles of chemotherapy are used, the surgical approach should be modified to permit less liver ischemia time during transection. Pringle maneuver is generally not used in transecting liver parenchyma with CUSA, ERBE, or TissueLink. In these cases where patients are at higher risk of liver failure use of these instruments may prove beneficial. Although the Cochrane database shows that no parenchymal technique is superior to the crush-clamp and vessel ligation technique in regards to bile leaks, bleeding, and liver failure, it does not specifically address the complications in relation to colorectal liver metastasis. There is literature to support an individual’s use of a preferred technique over another technique with low morbidity. The key point is repetition in one technique allows a surgeon to become very comfortable in that method. Subsequently, after the learning curve the rates of complications decrease. Due to the complexity of liver resections in colorectal liver metastasis, surgeons should be comfortable in utilizing different approaches. The radiofrequency ablation method provides sealing of biliary radicals and small vessels but not larger vasculature and should be used with caution during deeper dissection in the liver.
The open surgical approach to liver resection will continue to grow and develop with technology. With growing interest in minimally invasive approach to liver resection, this adds an additional tool to the technology available for open surgery. It is important for surgeons to understand that laparoscopy or the robot is another tool in performing liver resection. The gold standard will always be open surgery. Patient selection and surgeon experience are critically important to the success of minimally invasive liver resection. Due to the complexity of liver surgery, this approach should be reserved for specialized institutions that are involved in not only doing these cases routinely, but training other surgeons in minimally invasive technique as well.
Finally, it is important that surgeons understand the risks, benefits, and costs of various surgical approaches to liver resection for colorectal metastasis. In an era where focus is placed on cost savings in medicine, comparing the cost benefit of the different techniques in liver resection will play an important role in how we manage these patients.
Footnote
No potential conflict of interest.
References
- Blumgart LH, Belghiti J. Surgery of the liver, biliary tract, and pancreas. 4th ed. Philadelphia, PA: Saunders Elsevier, 2007.
- Skandalakis JE, Skandalakis LJ, Skandalakis PN, Mirilas P. Hepatic surgical anatomy. Surg Clin North Am 2004;84:413-435. viii.. [PubMed]
- Bismuth H. Surgical anatomy and anatomical surgery of the liver. World J Surg 1982;6:3-9. [PubMed]
- Couinaud C. Liver anatomy: portal (and suprahepatic) or biliary segmentation. Dig Surg 1999;16:459-467. [PubMed]
- Strasberg SM. Nomenclature of hepatic anatomy and resections: a review of the Brisbane 2000 system. J Hepatobiliary Pancreat Surg 2005;12:351-355. [PubMed]
- Vauthey JN, Pawlik TM, Ribero D, et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol 2006;24:2065-2072. [PubMed]
- Gold JS, Are C, Kornprat P, et al. Increased use of parenchymal-sparing surgery for bilateral liver metastases from colorectal cancer is associated with improved mortality without change in oncologic outcome: trends in treatment over time in 440 patients. Ann Surg 2008;247:109-117. [PubMed]
- van der Pool AE, Lalmahomed ZS, de Wilt JH, Eggermont AM, Ijzermans JM, Verhoef C. Local treatment for recurrent colorectal hepatic metastases after partial hepatectomy. J Gastrointest Surg 2009;13:890-895. [PubMed]
- Lalmahomed ZS, Ayez N, van der Pool AE, Verheij J, IJzermans JN, Verhoef C. Anatomical versus nonanatomical resection of colorectal liver metastases: is there a difference in surgical and oncological outcome? World J Surg 2011;35:656-661. [PubMed]
- Sarpel U, Bonavia AS, Grucela A, Roayaie S, Schwartz ME, Labow DM. Does anatomic versus nonanatomic resection affect recurrence and survival in patients undergoing surgery for colorectal liver metastasis? Ann Surg Oncol 2009;16:379-384. [PubMed]
- Zorzi D, Mullen JT, Abdalla EK, et al. Comparison between hepatic wedge resection and anatomic resection for colorectal liver metastases. J Gastrointest Surg 2006;10:86-94. [PubMed]
- Finch RJ, Malik HZ, Hamady ZZ, et al. Effect of type of resection on outcome of hepatic resection for colorectal metastases. Br J Surg 2007;94:1242-1248. [PubMed]
- Shimada M, Matsumata T, Akazawa K, et al. Estimation of risk of major complications after hepatic resection. Am J Surg 1994;167:399-403. [PubMed]
- Abdalla EK, Noun R, Belghiti J. Hepatic vascular occlusion: which technique? Surg Clin North Am 2004;84:563-585. [PubMed]
- Man K, Fan ST, Ng IO, Lo CM, Liu CL, Wong J. Prospective evaluation of Pringle maneuver in hepatectomy for liver tumors by a randomized study. Ann Surg 1997;226:704-711, discussion 711-713. [PubMed]
- Elias D, Desruennes E, Lasser P. Prolonged intermittent clamping of the portal triad during hepatectomy. Br J Surg 1991;78:42-44. [PubMed]
- Gurusamy KS, Sheth H, Kumar Y, Sharma D, Davidson BR. Methods of vascular occlusion for elective liver resections. Cochrane Database Syst Rev 2009;CD007632. [PubMed]
- Clavien PA, Yadav S, Sindram D, Bentley RC. Protective effects of ischemic preconditioning for liver resection performed under inflow occlusion in humans. Ann Surg 2000;232:155-162. [PubMed]
- Rahbari NN, Wente MN, Schemmer P, et al. Systematic review and meta-analysis of the effect of portal triad clamping on outcome after hepatic resection. Br J Surg 2008;95:424-432. [PubMed]
- Huguet C, Gavelli A, Chieco PA, et al. Liver ischemia for hepatic resection: where is the limit? Surgery 1992;111:251-259. [PubMed]
- Belghiti J, Noun R, Malafosse R, et al. Continuous versus intermittent portal triad clamping for liver resection: a controlled study. Ann Surg 1999;229:369-375. [PubMed]
- Man K, Fan ST, Ng IO, et al. Tolerance of the liver to intermittent pringle maneuver in hepatectomy for liver tumors. Arch Surg 1999;134:533-539. [PubMed]
- Delva E, Barberousse JP, Nordlinger B, et al. Hemodynamic and biochemical monitoring during major liver resection with use of hepatic vascular exclusion. Surgery 1984;95:309-318. [PubMed]
- Torzilli G, Makuuchi M, Midorikawa Y, et al. Liver resection without total vascular exclusion: hazardous or beneficial? An analysis of our experience. Ann Surg 2001;233:167-175. [PubMed]
- Belghiti J, Noun R, Zante E, Ballet T, Sauvanet A. Portal triad clamping or hepatic vascular exclusion for major liver resection. A controlled study. Ann Surg 1996;224:155-161. [PubMed]
- Lesurtel M, Selzner M, Petrowsky H, McCormack L, Clavien PA. How should transection of the liver be performed?: a prospective randomized study in 100 consecutive patients: comparing four different transection strategies. Ann Surg 2005;242:814-822, discussion 822-823. [PubMed]
- Rahbari NN, Koch M, Schmidt T, et al. Meta-analysis of the clamp-crushing technique for transection of the parenchyma in elective hepatic resection: back to where we started? Ann Surg Oncol 2009;16:630-639. [PubMed]
- Gurusamy KS, Pamecha V, Sharma D, Davidson BR. Techniques for liver parenchymal transection in liver resection. Cochrane Database Syst Rev 2009;CD006880. [PubMed]
- Rahbari NN, Elbers H, Koch M, et al. Clamp-crushing versus stapler hepatectomy for transection of the parenchyma in elective hepatic resection (CRUNSH)--a randomized controlled trial (NCT01049607). BMC Surg 2011;11:22. [PubMed]
- Poon RT. Current techniques of liver transection. HPB (Oxford) 2007;9:166-173. [PubMed]
- Fan ST, Lai EC, Lo CM, Chu KM, Liu CL, Wong J. Hepatectomy with an ultrasonic dissector for hepatocellular carcinoma. Br J Surg 1996;83:117-120. [PubMed]
- Kim J, Ahmad SA, Lowy AM, et al. Increased biliary fistulas after liver resection with the harmonic scalpel. Am Surg 2003;69:815-819. [PubMed]
- Patrlj L, Tuorto S, Fong Y. Combined blunt-clamp dissection and LigaSure ligation for hepatic parenchyma dissection: postcoagulation technique. J Am Coll Surg 2010;210:39-44. [PubMed]
- Saiura A, Yamamoto J, Koga R, et al. Usefulness of LigaSure for liver resection: analysis by randomized clinical trial. Am J Surg 2006;192:41-45. [PubMed]
- Ikeda M, Hasegawa K, Sano K, et al. The vessel sealing system (LigaSure) in hepatic resection: a randomized controlled trial. Ann Surg 2009;250:199-203. [PubMed]
- Geller DA, Tsung A, Maheshwari V, Rutstein LA, Fung JJ, Marsh JW. Hepatic resection in 170 patients using saline-cooled radiofrequency coagulation. HPB (Oxford) 2005;7:208-213. [PubMed]
- Lupo L, Gallerani A, Panzera P, Tandoi F, Di Palma G, Memeo V. Randomized clinical trial of radiofrequency-assisted versus clamp-crushing liver resection. Br J Surg 2007;94:287-291. [PubMed]
- Rau HG, Duessel AP, Wurzbacher S. The use of water-jet dissection in open and laparoscopic liver resection. HPB (Oxford) 2008;10:275-280. [PubMed]
- Pamecha V, Gurusamy KS, Sharma D, Davidson BR. Techniques for liver parenchymal transection: a meta-analysis of randomized controlled trials. HPB (Oxford) 2009;11:275-281. [PubMed]
- Schemmer P, Bruns H, Weitz J, Schmidt J, Büchler MW. Liver transection using vascular stapler: a review. HPB (Oxford) 2008;10:249-252. [PubMed]
- Schemmer P, Friess H, Dervenis C, et al. The use of endo-GIA vascular staplers in liver surgery and their potential benefit: a review. Dig Surg 2007;24:300-305. [PubMed]
- Reddy SK, Barbas AS, Gan TJ, Hill SE, Roche AM, Clary BM. Hepatic parenchymal transection with vascular staplers: a comparative analysis with the crush-clamp technique. Am J Surg 2008;196:760-767. [PubMed]
- Nguyen KT, Geller DA. Laparoscopic liver resection--current update. Surg Clin North Am 2010;90:749-760. [PubMed]
- Nguyen KT, Gamblin TC, Geller DA. World review of laparoscopic liver resection-2,804 patients. Ann Surg 2009;250:831-841. [PubMed]
- Giulianotti PC, Sbrana F, Coratti A, et al. Totally robotic right hepatectomy: surgical technique and outcomes. Arch Surg 2011;146:844-850. [PubMed]
- Hata S, Imamura H, Aoki T, et al. Value of visual inspection, bimanual palpation, and intraoperative ultrasonography during hepatic resection for liver metastases of colorectal carcinoma. World J Surg 2011;35:2779-2787. [PubMed]
- Cervone A, Sardi A, Conaway GL. Intraoperative ultrasound (IOUS) is essential in the management of metastatic colorectal liver lesions. Am Surg 2000;66:611-615. [PubMed]
- Hirokawa F, Hayashi M, Miyamoto Y, et al. Re-evaluation of the necessity of prophylactic drainage after liver resection. Am Surg 2011;77:539-544. [PubMed]
- Aldameh A, McCall JL, Koea JB. Is routine placement of surgical drains necessary after elective hepatectomy? Results from a single institution. J Gastrointest Surg 2005;9:667-671. [PubMed]
- Gurusamy KS, Samraj K, Davidson BR. Routine abdominal drainage for uncomplicated liver resection. Cochrane Database Syst Rev 2007;CD006232. [PubMed]