
Loss of ferrochelatase is protective against colon cancer cells: ferrochelatase a possible regulator of the long noncoding RNA H19
Introduction
Human ferrochelatase (FECH) is the last enzyme in the heme biosynthetic pathway. FECH is located in the inner mitochondrial membrane and catalyzes the conversion of protoporphyrin IX (PpIX) to heme (1,2). Deficiency in the FECH is associated with many diseases, including erythropoietic protoporphyria (3). The correlation between FECH and cancer has not been fully described. Studies showed that the oral administration of 5-aminolevulinic acid resulted in the progressive accumulation of protoporphyrin in a rat colon carcinoma but not in the surrounding liver tissue. These results suggest that the selective accumulation of porphyrins is caused by the relative FECH deficiency in the malignant tissue (4). It has been also shown that a transcriptional down-regulation of FECH occurs in malignant tissues, causing endogenous PpIX accumulation (2). Subsequently, a loss of FECH activity as well as decreased expression were detected in human carcinomas, and were responsible for PpIX accumulation. This outcome has led to the development of useful tools for detection and treatment of neoplastic diseases because PpIX is a fluorescing and photodynamically active chromophore and therefore targeting it may specifically destroy the cancerous but not the unaffected tissue (5-7).
In a previous study, we surveyed FECH expression patterns in colon cancer. Using publicly available microarray data sets, we found that FECH mRNA was largely and significantly decreased in colon adenocarcinomas (COAD) relative to normal colon tissues (8).
To investigate the potential role of FECH in tumorigenesis, we established an in vitro model comprising the use of a small interfering RNA (siRNA) to knockdown FECH in human Caco-2 colon cancer cells. The subsequent effect of FECH down-regulation was explored on cell proliferation, gene targets, long noncoding RNA (LncRNA) H19, and migration of cancer cells in comparison to human normal fibroblasts.
Methods
Cell culture
Caco-2 colon cancer cells, HT-29 colon cancer cells, C3A liver cancer cells, PC3 prostate cancer cells were originally purchased from American Type Culture Collection (ATCC). Human skin fibroblasts were derived from normal adherent cells originally obtained from a skin biopsy. Fibroblasts and cancer cells were cultured in DMEM medium (Sigma, USA) supplemented with 10% v/v fetal bovine serum (FBS) (Sigma, USA) with antibiotic solution [1% penicillin and streptomycin (Sigma, USA)]. Cells were kept at 37 °C in a humidified atmosphere of 5% CO2.
The study was approved by the Institutional Review Board of the American university of Beirut, Lebanon (IRB number: DER.MK.01) and informed consent was taken from the healthy individual prior to the skin biopsy.
FECH siRNA transfection
On the day of transfection, Caco-2 cells or human skin fibroblasts (60,000 cells/well) were seeded in a 24 well and incubated under normal growth conditions (37 °C and 5% CO2). Seventy-five ng of siRNA (TTCGGTACGTCCATCCTTTAA; SI03023090, Qiagen, Germany) were diluted in serum free medium, added to HiPerFect transfection reagent (Qiagen, Germany) and mixed for 10 min at room temperature. Transfection complexes were next added drop-wise onto the cells. Caco-2 cells and fibroblasts were divided into two groups: control (transfected with control siRNA) and FECH (transfected with FECH siRNA). Cells were incubated at 37 °C for 72 h without changing their medium.
Real time PCR (qPCR)
Caco-2 cells, HT-29 cells, C3A cells, PC3 cells and human fibroblasts were seeded on 6-well plates and transfected with siRNA of the FECH gene. Total RNA (1 µg) was isolated from fibroblasts and reverse-transcribed using a Superscript First-Strand cDNA synthesis kit (Invitrogen, USA) as per manufacturer’s protocol. The resulting cDNA was then subjected to qPCR (CFX96, Bio-Rad, USA) to quantify gene expression for FECH, vascular endothelial growth factor (VEGF), Snail, Twist, hypoxia induced factor (HIF-1α) and H19 using specific primers (Table 1). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control. Each sample was analyzed in triplicate. Fold changes were determined using the 2−ΔΔCt method.
Full table
MTT cell proliferation assay
The proliferation of Caco-2 cells and fibroblasts following siRNA transfection was detected using MTT assay. Briefly, 10,000 cells were plated on 96-well plates, transfected with siRNA of the FECH gene and cultured at 37 °C. After 72 h of transfection, 15 µL of MTT dye (Promega, USA) was added to each well and cells were incubated at 37 °C for 4 h. A total of 100 µL of stop solution was added to each well to dissolve the formazan crystals. Following incubation at 37 °C for 1 h, the optical density of each sample was recorded at a wavelength of 570 nm using the Multiskan Ex spectrum.
Cell cycle analysis
The effect of FECH knockdown on the cell cycle distribution of Caco-2 cells and fibroblasts was analyzed using propidium iodide (PI) (Sigma, USA) and flow cytometry. Following 72 h of transfection, suspension and adherent cells were collected, washed with ice cold phosphate buffered saline (PBS) and fixed in ethanol overnight. Cells were next washed with PBS and stained with PI (1 mg/mL) for 10 minutes after RNase A treatment (200 µg/mL). Analysis was performed using flow cytometer (GuavaEasy Cyte8 Flow cytometer, Luminex, USA).
Western blot analysis
Caco-2 cells and fibroblasts were seeded on 6-well plates and transfected using FECH siRNA. Cells were collected after 72 h of transfection and lysed on ice. The expression of FECH was detected by immunoblotting after resolving proteins using SDS-PAGE gels. Proteins were next transferred to PVDF membranes (Biorad, USA) and blocked with 5% milk in PBS-Tween (PBST). Membranes were then incubated with primary antibodies overnight at 4 °C: anti-FECH (1 µg/mL, Santa Cruz, USA) and anti-GAPDH (1 µg/mL, Santa Cruz, USA). After PBST washes, horseradish peroxidase linked secondary antibody was applied at 2 µg/mL for 1 h at room temperature. Bands were visualized using ECL chemoluminescent system (Biorad, USA) and quantified using ImageJ software (Wayne Rasband, USA).
Wound healing assay
Cellular migration was measured using a scratch assay. After 72 h of siRNA transfection, cells were treated with mitomycin C (10 µg/mL) for 40 min. The monolayer of Caco-2 cells and fibroblasts reaching 90% of confluency was wounded using a micropipette tip (200 µL tip), gently rinsed with PBS, and then incubated for additional 24 h at 37 °C and 5% CO2 humidified atmosphere. Caco-2 cells and fibroblasts migration was photographed using a microscopy (Zeiss Axio Observer Z1, Germany) at 0 h (time of the scratch) and 24 h with or without H2O2 (50 µM). Cell migration was quantified from 3 separate experiments measuring at least two scratches per condition. Wound diameter was measured using Image J software (Wayne Rasband, USA).
In silico analysis
To analyze the prognostic value of FECH, we used the gepia platform (9) on patient samples with COAD from the cancer genome atlas (TCGA). The patient samples were split into two groups (n=135 for each group) according to a median quantile expression cut-off (50%). The two patient cohorts were compared by a Kaplan-Meier survival plot, and the hazard ratio (HR) with 95% confidence intervals and logrank P value was calculated using the Cox regression model.
Results
FECH gene was down-regulated in Caco-2 colon cancer cells
To investigate the potential role of FECH in colon cancer, down-regulation of the gene was performed in Caco-2 cells, as an example of colon cancer cell line, and compared to skin fibroblasts, as a normal cell counterpart.
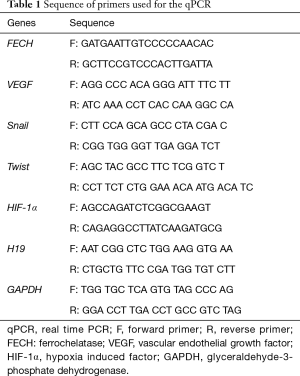
Exponentially growing Caco-2 colon cancer cells were transfected with siRNA of the FECH gene in order to down-regulate the expression of this protein. qPCR analysis showed that the mRNA expression levels of FECH were significantly down-regulated by 85% after 72 h of siRNA transfection when compared to cells treated with control siRNA (Figure 1A). In parallel, FECH was successively down-regulated to 60% in primary fibroblasts isolated from healthy individual (Figure 1B). Down-regulation of FECH was further confirmed at the protein level by western blot analysis. Results showed that FECH expression in Caco-2 cancer cells was reduced by 58% when compared to control (Figure 1C). In the same context, the level of FECH protein was also decreased by 56% (Figure 1D) in comparison to the protein levels in control fibroblasts.
FECH silencing was not cytotoxic to Caco-2 colon cancer cells
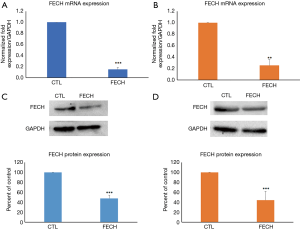
To assess whether FECH silencing is cytotoxic to cells, proliferation assays were conducted on Caco-2 cells and normal fibroblasts. The viability of Caco-2 cells was evaluated 72 h post-transfection using the MTT assay. Results demonstrated that the proliferation of Caco-2 cells was not affected following FECH down-regulation (Figure 2A). In addition, cell cycle analysis revealed that FECH silencing induced a slight increase in the pre-G0-G1 levels, but with no effect on the cycle of Caco-2 cells (Figure 2B). Fibroblasts were more sensitive to the down-regulation of FECH gene, where their proliferation was reduced to 30%, as compared to control cells (Figure 2C). However, the cell cycle analysis didn’t show any dysregulation following FECH down-regulation (Figure 2D).
Taken together, FECH down-regulation didn’t affect the proliferation of Caco-2 cancer cells and fibroblasts which suggests that FECH is not an essential gene for the viability of these cells.
FECH silencing decreased Snail, Twist, VEGF and HIF-1α expression in Caco-2 cancer cells
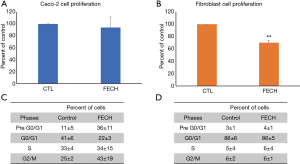
In order to investigate whether FECH has a protecting or a promoting role in the tumorigenesis of colon cancer, we examined the effect of FECH silencing on the mRNA expression of epithelial to mesenchymal transition (EMT) markers, VEGF and HIF-1α. Results demonstrated that the expression of Twist and Snail decreased to 0.1 folds in Caco-2 cells when compared to control cells. Furthermore, HIF-1α expression, was reduced to 0.08 folds and subsequently the expression of VEGF was reduced to 0.3 folds in Caco-2 cells, when compared to the control (Figure 3A). Conversely, VEGF and HIF-1α were both upregulated by up to 2 folds in fibroblasts after FECH silencing (Figure 3B). It is noteworthy that these latter proteins are interconnected to the hypoxia-induced pathway.
These results suggest that the presence of FECH confer a mesenchymal-like phenotype to cancer cells. Furthermore, FECH might be a positive regulator protein for the hypoxia pathway, as its loss is simultaneously causing a reduction in the expression of HIF-1α and VEGF.
FECH silencing suppressed H19 expression in Caco-2 cancer cells
H19 is a LncRNA, involved in the tumorigenesis of many types of cancer. Recent studies demonstrated that H19 was upregulated in colon cancer tissues compared to adjacent noncancerous tissues (10). Moreover, the overexpression of H19 promoted colon cancer cell proliferation by competitively binding to miR-200a and inhibiting β-catenin (11). We investigated the expression of H19 in Caco-2 cells and compared it to different cancer cells including HT-29 colon cancer cells, C3A liver cancer cells, PC3 prostate cancer cells and normal fibroblasts (Figure 4A). Results showed that H19 was 70% down-regulated following FECH silencing in Caco-2 cells, when compared to controls (Figure 4B). However, no effect on H19 expression was observed in normal fibroblasts after FECH knockdown (Figure 4C).
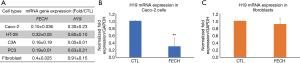
Altogether, our results suggest that H19 might be one of FECH targets in colon cancer cells. Thus, a better prognosis of colon cancer could be directly linked to the loss of FECH and subsequently decreased levels of H19.
FECH silencing combined with H2O2 insult promoted the migration of Caco-2 cancer cells
Tissue invasion and metastases are key phenotypic characteristics of cancer cells. Therefore, we assessed the role of FECH on cellular migration, a key determinant of malignant progression and metastases. Wound healing assay was performed on Caco-2 cells down-regulated FECH and compared to normal fibroblasts. No difference in the migration was found between FECH knockdown and control conditions in Caco-2 cancer cells (Figure 5A). An additional stress consisting of H2O2 insult in Caco-2 cells was performed prior to the assessment of the cellular migration. Results showed that the diameter of the wound was around 40% lower in FECH down-regulated condition, in comparison to control cells. Consequently, a significant increase in cell migration was found in FECH knockdown treated with H2O2 (Figure 5B). Interestingly, the same results were observed in fibroblasts in the absence of H2O2 (Figure 5C), whereas a slight delay (14%) in the cellular migration was scored in the presence of H2O2 after concomitant down-regulation of FECH gene (Figure 5D).
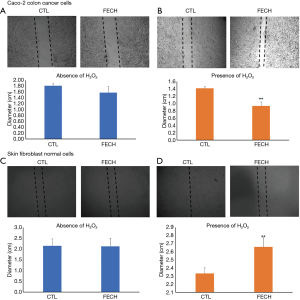
These results suggest that FECH alone was not able to change the migration ability of cells. Thus, at least two events must be dysregulated in Caco-2 cells to promote their motility.
FECH levels in colon tissues were correlated with survival of colon cancer patients
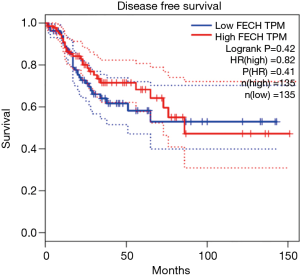
The implication of FECH in the tumorigenesis of colon cancer remain elusive. Using available datasets, correlation studies were carried out on colon cancer patients having high (n=135) and low (n=135) FECH expression in their colon tissue. Results demonstrated that the group with low FECH has an overall better survival rate. Specifically, the disease-free survival curve showed that patients with low FECH levels have ~1.25× higher chance to survive than patients with high FECH levels, for up to 150 months follow up (Figure 6). These relationships suggest that FECH loss might be beneficial for patients having colon cancer.
Discussion
Our results suggest that loss of FECH might be protective against colon cancer. We demonstrated that FECH silencing reduced the mesenchymal pro-tumorigenic phenotype and inhibited the HIF-1α pathway in Caco-2 colon cancer cells. Interestingly, we showed that H19, a LncRNA implicated in increased tumor severity as well as metastasis, might be a target of FECH in cancer as its levels markedly decreased upon the knockdown of FECH.
The role of FECH in tumorigenesis is not well established; however, some indirect indicators exist. For instance, medications composed of acetylsalicylic acid such as aspirin, have been shown to be protective in different cancers including colon cancer, prostate cancer and bladder cancer (12,13). Interestingly, these medications induce mitochondrial injury by directly inhibiting the activity of FECH (14). In the absence of FECH, aspirin effect will probably be abolished in the mitochondria. Therefore, it is possible that the initial role of aspirin in the prevention of these cancers is through inhibiting the action of FECH.
Moreover, it was recently shown that dormant cancer cells accumulate PpIX, and thus it is possible that loss of FECH will induce/maintain or at least contribute to cancer dormancy (15). Dormant cancers are resistant to both chemotherapy and immunotherapy and thus targeting PpIX in these cells may offer a potential novel therapeutic window.
Additionally, a recent study showed that PpIX is involved in the RAS signaling pathway, which is implicated in a significant number of cancers (16). Mitogen-activated protein kinase (MEK), a downstream mediator of RAS, was shown to regulate the heme biosynthetic pathway, where inhibition of MEK leads to the accumulation of PpIX (16). Finally, PpIX has recently been the target for therapy in many tumors including malignant brain gliomas and bladder cancer (17-20).
Our results demonstrated that the FECH gene and the HIF-1α pathway are connected in cancer and normal conditions. While FECH down-regulation decreased HIF-1α expression levels in Caco-2 cells, an upregulation of the gene was observed in normal fibroblasts. Previous studies showed that two HIF-1 binding motifs were identified within the FECH minimal promoter sequence. Exposure of cells to hypoxia resulted in an increase in FECH mRNA expression and a transactivation of the minimal promoter for the FECH gene (21). However, in normal conditions, the pathway might be overridden. Qiao et al. showed that miR-210 (another regulator of FECH) reduced cellular heme levels and the cytosolic heme-containing proteins in normal cardiomyocyte cells by modulating FECH only under normoxia (22). Altogether, these results suggest that during hypoxia in cancer cells, not only the FECH gene is a target for HIF-1α, but a possible feedback mechanism implicating HIF-1α as a downstream protein of the FECH gene might exists.
The LncRNA-H19 plays a major role in colon tumorigenesis as well as progression. High levels of H19 promote EMT and accelerate tumor growth both in vitro and in vivo as well as increased in tumor cell viability and migration (10,23). Moreover, survival rates were highly decreased in individuals whose cancers retained higher levels of H19 expression. Recently, it was found that H19 promotes migration and invasion of colon cancer through the RAS-MAPK signaling pathway (24).
Here we showed that loss of FECH resulted in decreased levels of HIF-1α and VEGF as well as decreased levels of mesenchymal stem markers such as Twist and Snail. Additionally, a significant decrease in H19 levels was detected not only in colon cancer cells (Caco-2 and HT-29), but also the effect was reproduced in C3A liver cancer cells and PC3 prostate cancer cells. Thus, our data suggest that FECH is a potential regulator of H19 and targeting it may offer a potential treatment for colon cancer as well as other cancers. This regulation might be through the RAS signaling pathway as both seem to be players in this process.
In conclusion, we showed that loss of FECH is protective against tumorigenesis in vitro and this effect could be in part be mediated through inhibition of H19. Further work will be needed to better understand the relationship between FECH and H19. The implications of this work may carry the potential of additional therapeutic modalities for colon cancer.
Acknowledgments
We would like to thank all the individuals who helped in completing this work including Mrs. Inaam El Rassi. This work is supported by a grant from the American University of Beirut MPP and URB to Mazen Kurban.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Institutional Review Board of the American university of Beirut, Lebanon (IRB number: DER.MK.01) and informed consent was taken from the healthy individual prior to the skin biopsy.
References
- Ferreira GC, Franco R, Lloyd SG, et al. Structure and function of ferrochelatase. J Bioenerg Biomembr 1995;27:221-9. [Crossref] [PubMed]
- Kemmner W, Wan K, Rüttinger S, et al. Silencing of human ferrochelatase causes abundant protoporphyrin-IX accumulation in colon cancer. FASEB J 2008;22:500-9. [Crossref] [PubMed]
- Taketani S, Fujita H. The ferrochelatase gene structure and molecular defects associated with erythropoietic protoporphyria. J Bioenerg Biomembr 1995;27:231-8. [Crossref] [PubMed]
- Van Hillegersberg R, Van den Berg JW, Kort WJ, et al. Selective accumulation of endogenously produced porphyrins in a liver metastasis model in rats. Gastroenterology 1992;103:647-51. [Crossref] [PubMed]
- Inoue K, Ota U, Ishizuka M, et al. Porphyrins as urinary biomarkers for bladder cancer after 5-aminolevulinic acid (ALA) administration: the potential of photodynamic screening for tumors. Photodiagnosis Photodyn Ther 2013;10:484-9. [Crossref] [PubMed]
- Nakai Y, Tatsumi Y, Miyake M, et al. Expression of ferrochelatase has a strong correlation in protoporphyrin IX accumulation with photodynamic detection of bladder cancer. Photodiagnosis Photodyn Ther 2016;13:225-32. [Crossref] [PubMed]
- Teng L, Nakada M, Zhao SG, et al. Silencing of ferrochelatase enhances 5-aminolevulinic acid-based fluorescence and photodynamic therapy efficacy. Br J Cancer 2011;104:798-807. [Crossref] [PubMed]
- Kadara H, Nemer G, Safi R, et al. Erythropoietic protoporphyria a clinical and molecular study from Lebanon: Ferrochelatase a potential tumor suppressor gene in colon cancer. Clin Genet 2017;92:495-502. [Crossref] [PubMed]
- Tang Z, Li C, Kang B, et al. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res 2017;45:W98-102. [Crossref] [PubMed]
- Chen SW, Zhu J, Ma J, et al. Overexpression of long non-coding RNA H19 is associated with unfavorable prognosis in patients with colorectal cancer and increased proliferation and migration in colon cancer cells. Oncol Lett 2017;14:2446-52. [Crossref] [PubMed]
- Yang W, Ning N, Jin X. The lncRNA H19 Promotes Cell Proliferation by Competitively Binding to miR-200a and Derepressing β-Catenin Expression in Colorectal Cancer. Biomed Res Int 2017;2017:2767484.
- Pathi S, Jutooru I, Chadalapaka G, et al. Aspirin inhibits colon cancer cell and tumor growth and downregulates specificity protein (Sp) transcription factors. PLoS One 2012;7:e48208. [Crossref] [PubMed]
- Ruder EH, Laiyemo AO, Graubard BI, et al. Non-steroidal anti-inflammatory drugs and colorectal cancer risk in a large, prospective cohort. Am J Gastroenterol 2011;106:1340-50. [Crossref] [PubMed]
- Gupta V, Liu S, Ando H, et al. Salicylic acid induces mitochondrial injury by inhibiting ferrochelatase heme biosynthesis activity. Mol Pharmacol 2013;84:824-33. [Crossref] [PubMed]
- Nakayama T, Otsuka S, Kobayashi T, et al. Dormant cancer cells accumulate high protoporphyrin IX levels and are sensitive to 5-aminolevulinic acid-based photodynamic therapy. Sci Rep 2016;6:36478. [Crossref] [PubMed]
- Yoshioka E, Chelakkot VS, Licursi M, et al. Enhancement of Cancer-Specific Protoporphyrin IX Fluorescence by Targeting Oncogenic Ras/MEK Pathway. Theranostics 2018;8:2134-46. [Crossref] [PubMed]
- Inoue K. 5-Aminolevulinic acid-mediated photodynamic therapy for bladder cancer. Int J Urol 2017;24:97-101. [Crossref] [PubMed]
- Nakai Y, Ozawa T, Mizuno F, et al. Spectrophotometric photodynamic detection involving extracorporeal treatment with hexaminolevulinate for bladder cancer cells in voided urine. J Cancer Res Clin Oncol 2017;143:2309-16. [Crossref] [PubMed]
- Schwake M, Nemes A, Dondrop J, et al. In-Vitro Use of 5-ALA for Photodynamic Therapy in Pediatric Brain Tumors. Neurosurgery 2018;83:1328-37. [Crossref] [PubMed]
- Yoneda T, Nonoguchi N, Ikeda N, et al. Spectral Radiance of Protoporphyrin IX Fluorescence and Its Histopathological Implications in 5-Aminolevulinic Acid-Guided Surgery for Glioblastoma. Photomed Laser Surg 2018;36:266-72. [Crossref] [PubMed]
- Liu YL, Ang SO, Weigent DA, Prchal JT, Bloomer JR. Regulation of ferrochelatase gene expression by hypoxia. Life Sci 2004;75:2035-43. [Crossref] [PubMed]
- Qiao A, Khechaduri A, Kannan Mutharasan R, et al. MicroRNA-210 decreases heme levels by targeting ferrochelatase in cardiomyocytes. J Am Heart Assoc 2013;2:e000121. [Crossref] [PubMed]
- Liang WC, Fu WM, Wong CW, et al. The lncRNA H19 promotes epithelial to mesenchymal transition by functioning as miRNA sponges in colorectal cancer. Oncotarget 2015;6:22513-25. [Crossref] [PubMed]
- Yang W, Redpath RE, Zhang C, et al. Long non-coding RNA H19 promotes the migration and invasion of colon cancer cells via MAPK signaling pathway. Oncol Lett 2018;16:3365-72. [PubMed]


