
Safety and efficacy of locoregional therapy for metastatic pancreatic ductal adenocarcinoma to the liver: a single-center experience
Introduction
PDAC is the fourth leading cause of cancer death in both men and women in the United States. Approximately half of patients present with advanced metastatic disease at diagnosis with a 5-year survival of 3% (1).
Chemotherapy is the mainstay of treatment for patients with metastatic disease, but nearly 40% of patients experience major side effects leading to decreased quality of life and treatment intolerance (2-5). Median overall survival (mOS) for systemic therapy under trial conditions ranges from 8.5–11 months (4-6). Radiation therapy, either concurrent chemoradiation or in sequence, provides effective primary local tumor control with an indeterminate survival benefit (7,8).
Pancreaticoduodenectomy (PDX) is the only potentially curative therapy for early stage pancreatic adenocarcinoma. R0 resections are subject to over an 80% risk of locoregional recurrence with median survival under trial conditions for non-metastatic disease between 17.9 and 23.6 months (9). Few studies have assessed the role of hepatic metastasectomy for PDAC with conflicting results that demonstrate unclear survival benefit (10-17).
With the advent of interventional oncology (IO), there has been increasing interest in the utilization of image-guided locoregional therapy (LRT) for the treatment of PDAC with hepatic metastasis (mPDAC). Radiofrequency ablation (RFA), cryoablation, irreversible electroporation (18), and microwave ablation (MWA) have all demonstrated efficacy and low morbidity (19,20). Transarterial chemoembolization (TACE) has also been investigated with demonstrable correlation in survival with local tumor imaging response and reported mOS of 9–19 months (21-23). Trans-arterial radioembolization (TARE) has been successfully used in metastatic pancreatic adenocarcinoma with a mOS of 9–22 months (24,25). The utilization of individual interventional oncologic modalities can vary greatly based on tumor morphology, anatomy, hepatic reserve, and institutional preference. This heterogeneous manifestation of disease and equally variable implementation of locoregional technology can be challenging to analyze. Nevertheless, many institutions offer LRT with a qualitatively perceived benefit in patients who would otherwise have limited options for mPDAC. We performed a retrospective evaluation of patients with mPDAC undergoing image-guided LRT to evaluate the safety and efficacy of this approach.
Methods
This study received institutional review board approval. A retrospective analysis of all LRTs performed for mPDAC by the Interventional Radiology (IR) Division between 01/2006-08/2017 was performed. Patient identification was accomplished using the Illuminate InSight search engine and its inherent natural language processing capabilities (Softek Illuminate, Inc., Overland Park, KS).
Analysis of the medical record was performed for all included patients. Patient demographics, extent of disease at diagnosis, prior chemotherapy, prior radiotherapy, prior or subsequent surgical therapy, and Eastern Cooperative Oncology Group (ECOG) performance status were collected. All patients underwent clinic evaluation and consultation in IR clinic prior to treatment. Data was collected for OS, survival since hepatic metastasis, and survival since LRT. For patients receiving multiple interventions, survival was calculated from the date of the initial intervention. Reported lab values were recorded for time points immediately prior to intervention as well as 3 and 6 months following intervention. Procedure related adverse events (AE) and lab value elevations were classified using Common Terminology Criteria for Adverse Events v5.0 (CTCAE 5.0).
Available imaging results for each patient were retrospectively reviewed by a board certified radiologist with fellowship training in abdominal imaging. Magnetic resonance imaging data was used when available. Imaging data was collected at baseline, as well as at 1, 3, and 6 months post-intervention, and every three months thereafter. Responses were defined as in-field (within the treatment liver volume) or out-of-field (outside of the treated liver volume). Both target lesion response and systemic response were graded using the modified Response Evaluation Criteria in Solid Tumors (mRECIST). mRECIST was modified for use with portal venous or delayed phase imaging, since mPDAC is typically not arterially hyperenhancing like hepatocellular carcinoma, and is more conspicuous on later phases of contrast. PET imaging was not available for enough patients to contribute to the analysis.
Results
Twenty patients, 11 females and 9 males, were included in the study (Table 1). All patients had a prospectively documented ECOG score of 0 or 1. The majority of patients had undergone prior chemotherapy and surgical therapy. Four patients underwent multiple IR interventions (maximum 3). One patient initiated chemotherapy concurrently with intervention. One patient had prior stereotactic body radiation therapy to a site of liver metastasis and 8 patients had external radiation therapy to the primary tumor or surgical bed. Twelve patients underwent primary tumor resection, and in two cases, resection was performed after LRT of hepatic metastasis.
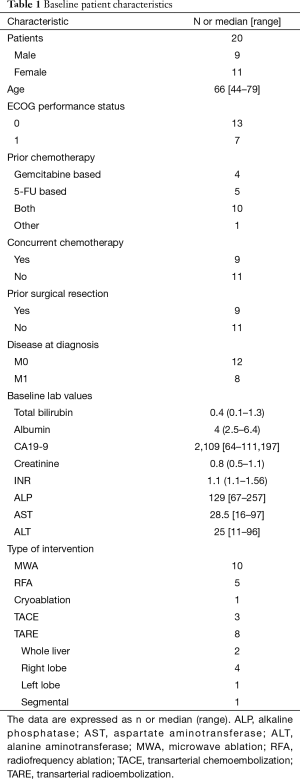
Full table
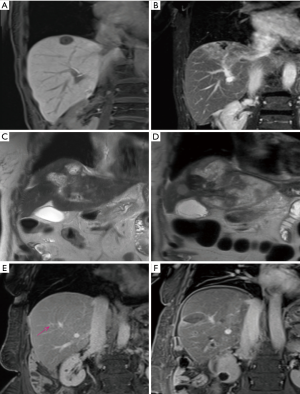
The type of LRT performed varied and was chosen by the treating physician based on individual patient characteristics in addition to tumor phenotype and distribution. Ablative techniques including thermal ablation and segmental ablative TARE [>190 Gy medical internal radiation dose (MIRD) using glass microspheres] were favored for well consolidated small lesions (Figure 1). Regional arterial therapies with TACE (Oncozene, Boston Scientific, 50–75 mg doxorubicin) and palliative TARE (<120 Gy MIRD for glass microspheres or body surface area dosimetry for resin microspheres) were used for multifocal disease.
Sixteen of twenty patients were treated with the intent of controlling limited volume hepatic disease. Two of these patients later received PDX, with one undergoing synchronous hepatic wedge resection of the ablation site and the other undergoing wedge resection 5 months later. The four remaining patients were treated to palliate extensive and symptomatic liver disease using TARE or TACE. Among patients treated with TARE, 2 received bilobar administration, 5 unilobar, and 1 segmental. Of the 20 patients, 9 underwent intervention concurrently with chemotherapy. LRT was timed between chemotherapy administrations, or in some cases, chemotherapy administration was delayed by 1–2 weeks to accommodate LRT.
Follow up imaging was available at 1 month for 17 patients, at 3 months for 9 patients, and at 6 months for 6 patients (Table 2). In-field lesion response was complete response (CR) or partial response (PR) by mRECIST criteria in 12 of 17 (70.5%) and 7 of 9 (77.7%) patients at 1 and 3 months, respectively. Out-of-field response was characterized as CR or PR in 3 of 17 (17.6%) and 1 of 9 (11.1%) patients at 1 and 3 months, respectively (Table 2). mOS from diagnosis for the entire study group was 25 months (range, 3.5–52 months). mOS following diagnosis of hepatic metastasis was 16.25 months (range, 2.5–39 months). mOS following intervention was 9.7 months (range, 0.75–37 months). When patients were stratified according to presence of response (CR and PR) of the in-field lesions vs. absence of response [stable disease (SD) and progressive disease (PD)] at 1 month post-intervention, median survival was 9 vs. 6 months (P=0.08). Median survival in responders vs. non-responders at 3 months was 11 vs. 7.75 months (P=0.07). Three patients were alive at the time of analysis, and two patients were lost to follow up at 5 and 3.5 months following diagnosis of hepatic disease. CA19-9 levels were available for 9 patients 3 months after treatment and were decreased from baseline (49–94%) in 6 of those patients.
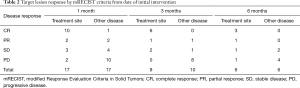
Full table
There were four grade 1 and two grade 3 AEs by CTCAE v5.0 criteria. Four patients experienced grade 1 pain and/or nausea managed at home. A grade 3 elevation of alkaline phosphatase occurred in one patient 2 months after TACE and was related to an episode of cholangitis. One patient developed post-embolization syndrome following TACE and required <72 hours supportive care and a 1–2-week delay in resumption of chemotherapy. A second patient developed transient acute renal failure with myoglobinuria following hepatic MWA and required temporary dialysis. The definitive cause of renal failure in this patient was never established, and the patient never received IV contrast.
Discussion
The prognosis of PDAC with distant metastasis is universally poor, with 5-year survival rates of less than 3% (1). Despite advances in chemotherapy, significant improvements in survival have been slow to emerge. There is mounting evidence for LRT to play a role in the care of patients with mPDAC. Surgical resection has been shown to be safe with minimal increase in morbidity when carried out synchronously with primary tumor resection (12,13,15,17). However, given the large proportion of patients who are not surgical candidates or who develop metachronous disease, LRT is an appealing alternative.
Park et al. studied 34 patients who underwent treatment of hepatic metastases with RFA immediately after or during pancreatectomy. Median survival after diagnosis of metastatic disease was 14 months. Kim et al. retrospectively reviewed 15 patients who underwent TACE after hepatic recurrence following curative resection with survival times following diagnosis of hepatic disease and initial TACE of 9.6 and 7.5 months, respectively (22). Vogl et al. studied 69 patients who received TACE with a combination of mitomycin C, cisplatin, and gemcitabine reporting a mOS of 19 months. In addition, they demonstrated an 11 month increase in survival among patients who had PR per RECIST criteria (21). In a retrospective review of 16 patients who underwent TARE concurrently with chemotherapy, Kim et al. demonstrated a median survival from diagnosis of metastatic disease and receipt of initial TARE of 22 and 12.5 months, respectively (24).
Our data demonstrate that LRT of mPDAC has an excellent safety profile in appropriately selected patients. LRT can also be performed without disruption of chemotherapy in the majority of patients. Among our 20 patients, there were two Grade 3 AEs and one chemotherapy-limiting toxicity. The mOS of 9.7 months following LRT is comparable to other modality-specific reports in the available literature. The low morbidity of LRT makes it widely applicable among high-performing patients. Notably, two patients in our study received PDX following ablation therapy of solitary hepatic metastases. These patients had OS of 30.5 and 38 months and survival post LRT of 29.5 and 37 months, respectively. While it is difficult to draw conclusions from these two cases alone, the possibility of down-staging mPDAC patients with favorable biology in order to undergo PDX is an exciting topic for future research efforts.
Several limitations are inherently present in this retrospective analysis. Our sample size of 20 patients is small, and limited conclusions can therefore be drawn from survival data. Due to the retrospective nature of the study, there is no control group for comparison. The presentation of mPDAC can be highly variable in both biology and anatomic involvement and the most appropriate locoregional treatment is tailored to these charactersitics. As such, retrospective analysis of mPDAC patients who receive LRT is inherently heterogeneous with various prior chemotherapy regimens and types of LRT. There is strong selection bias given the excellent performance status of our patients. The retrospective nature of this study did not allow for analysis of the effect of intent-to-treat. Referral practices and work-flow patterns have changed over the last 15 years. The creation of a formal Interventional Oncology clinic in the last few years will enable future acquisition of more meaningful statistics that cannot be applied to the series presented herein. While our study was not sufficiently powered to demonstrate statistical significance based on in-field lesion response, there was a strong trend toward improved survival, consistent with findings in the literature (21). Our patient-tailored application of variable LRT techniques demonstrated an excellent safety profile with high imaging response rate and did not disrupt systemic therapy. In light of the mounting retrospective evidence, prospective trials are warranted to definitively establish the role for LRTs.
Conclusions
The use of LRT in the treatment of metastatic pancreatic cancer is safe and does not significantly limit chemotherapy. Responders in our study demonstrated a trend toward improved survival. Further prospective evaluations are warranted.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study received institutional review board approval.
References
- Cancer Facts and Figures 2018. American Cancer Society 2018.
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-25. [Crossref] [PubMed]
- Peddi PF, Lubner S, McWilliams R, et al. Multi-institutional experience with FOLFIRINOX in pancreatic adenocarcinoma. JOP 2012;13:497-501. [PubMed]
- Portal A, Pernot S, Tougeron D, et al. Nab-paclitaxel plus gemcitabine for metastatic pancreatic adenocarcinoma after Folfirinox failure: an AGEO prospective multicentre cohort. Br J Cancer 2015;113:989-95. [Crossref] [PubMed]
- Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691-703. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology: Pancreatic Adenocarcinoma. (Version 3.2017). Available online: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf, accessed 1/12/18.
- Lischalk JW, Burke A, Chew J, et al. Five-Fraction Stereotactic Body Radiation Therapy (SBRT) and Chemotherapy for the Local Management of Metastatic Pancreatic Cancer. J Gastrointest Cancer 2018;49:116-23. [Crossref] [PubMed]
- Chang DT, Schellenberg D, Shen J, et al. Stereotactic radiotherapy for unresectable adenocarcinoma of the pancreas. Cancer 2009;115:665-72. [Crossref] [PubMed]
- Tachezy M, Gebauer F, Petersen C, et al. Sequential neoadjuvant chemoradiotherapy (CRT) followed by curative surgery vs. primary surgery alone for resectable, non-metastasized pancreatic adenocarcinoma: NEOPA- a randomized multicenter phase III study (NCT01900327, DRKS00003893, ISRCTN82191749). BMC Cancer 2014;14:411. [Crossref] [PubMed]
- Adam R, Chiche L, Aloia T, et al. Hepatic resection for noncolorectal nonendocrine liver metastases: analysis of 1,452 patients and development of a prognostic model. Ann Surg 2006;244:524-35. [PubMed]
- Crippa S, Bittoni A, Sebastiani E, et al. Is there a role for surgical resection in patients with pancreatic cancer with liver metastases responding to chemotherapy? Eur J Surg Oncol 2016;42:1533-9. [Crossref] [PubMed]
- Shrikhande SV, Kleeff J, Reiser C, et al. Pancreatic resection for M1 pancreatic ductal adenocarcinoma. Ann Surg Oncol 2007;14:118-27. [Crossref] [PubMed]
- Seelig SK, Burkert B, Chromik AM, et al. Pancreatic resections for advanced M1-pancreatic carcinoma: the value of synchronous metastasectomy. HPB Surg 2010;2010:579672. [Crossref] [PubMed]
- Yamada S, Fujii T, Sugimoto H, et al. Pancreatic cancer with distant metastases: a contraindication for radical surgery? Hepatogastroenterology 2009;56:881-5. [PubMed]
- Klein F, Puhl G, Guckelberger O, et al. The impact of simultaneous liver resection for occult liver metastases of pancreatic adenocarcinoma. Gastroenterol Res Pract 2012;2012:939350. [Crossref] [PubMed]
- Gleisner AL, Assumpcao L, Cameron JL, et al. Is resection of periampullary or pancreatic adenocarcinoma with synchronous hepatic metastasis justified? Cancer 2007;110:2484-92. [Crossref] [PubMed]
- Nikfarjam M, Sehmbey M, Kimchi ET, et al. Additional organ resection combined with pancreaticoduodenectomy does not increase postoperative morbidity and mortality. J Gastrointest Surg 2009;13:915-21. [Crossref] [PubMed]
- Narayanan G, Hosein PJ, Beulaygue IC, et al. Percutaneous Image-Guided Irreversible Electroporation for the Treatment of Unresectable, Locally Advanced Pancreatic Adenocarcinoma. J Vasc Interv Radiol 2017;28:342-8. [Crossref] [PubMed]
- Park JB, Kim YH, Kim J, et al. Radiofrequency ablation of liver metastasis in patients with locally controlled pancreatic ductal adenocarcinoma. J Vasc Interv Radiol 2012;23:635-41. [Crossref] [PubMed]
- Brown DB, Narayanan G. Interventional radiology and the pancreatic cancer patient. Cancer J 2012;18:591-601. [Crossref] [PubMed]
- Vogl TJ, Mohamed SA, Albrecht MH, et al. Transarterial chemoembolization in pancreatic adenocarcinoma with liver metastases: MR-based tumor response evaluation, apparent diffusion coefficient (ADC) patterns, and survival rates. Pancreatology 2018;18:94-9. [Crossref] [PubMed]
- Kim JH, Choi EK, Yoon HK, et al. Transcatheter arterial chemoembolization for hepatic recurrence after curative resection of pancreatic adenocarcinoma. Gut Liver 2010;4:384-8. [Crossref] [PubMed]
- Brown DB, Gonsalves CF, Yeo CJ, et al. One year survival with poorly differentiated metastatic pancreatic carcinoma following chemoembolization with gemcitabine and cisplatin. Oncol Rep 2010;24:767-9. [Crossref] [PubMed]
- Kim AY, Unger K, Wang H, et al. Incorporating Yttrium-90 trans-arterial radioembolization (TARE) in the treatment of metastatic pancreatic adenocarcinoma: a single center experience. BMC Cancer 2016;16:492. [Crossref] [PubMed]
- Michl M, Haug AR, Jakobs TF, et al. Radioembolization with Yttrium-90 microspheres (SIRT) in pancreatic cancer patients with liver metastases: efficacy, safety and prognostic factors. Oncology 2014;86:24-32. [Crossref] [PubMed]


