Hypercalcemia from metastatic pancreatic neuroendocrine tumor secreting 1,25-dihydroxyvitamin D
Case report
A 43-year-old African American woman with past medical history significant for a living related left kidney transplant in May 2004 for end stage renal disease of unclear etiology presented with shortness of breath in February 2008 and was incidentally found to have multiple round hypoattenuating lesions within the liver by CT scan of the thorax. A follow-up MRI scan of the abdomen showed a pancreatic mass with central necrosis, along with multiple masses in the liver with central necrosis. Ultrasound guided biopsy of a hypoechoic lesion within the right lobe of the liver revealed metastatic neuroendocrine tumor with positive staining for chromogranin, synaptophysin, cytokeratins AE1:3, CAM5.2, and CK7 and negative staining for CK20 and TTF-1. No mitotic figures were found in cytology or cell block slides, and the Ki-67 index was 2-5%. The above supported the diagnosis of well-differentiated metastatic neuroendocrine tumor (Figure 1A-C).
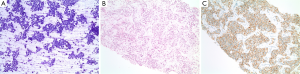
In June 2008, monthly octreotide was initiated and her immunosuppressant tacrolimus which had been started since the renal transplant was switched to sirolimus for better mTOR inhibition. Her disease had been stable until August 2010 when an MRCP showed significant enlargement of the known liver lesions with a stable pancreatic head lesion. The octreotide dose was increased from 20 to 30 mg. This, however, did not slow the progression and she subsequently underwent supra-selective hepatic chemoembolization of branches of the right and left hepatic artery with 75 mg of doxorubicin in March 2011. She had protracted anorexia and fatigue after the procedure likely resulting from major tumor necrosis suggested by the scan. She was again hospitalized 6 weeks later for fever and abdominal pain and was found to have Escherichia coli bacteremia secondary to ascending cholangitis from choledocholithiasis, which was successfully managed by ERCP with biliary sphincterotomy and stone extraction in addition to intravenous antibiotics. Although octreotide can cause cholelithiasis, it was resumed after this acute episode had subsided as the potential benefit was felt to outweigh this risk. Of note, a repeat ERCP with stone extraction was performed approximately nine months later.
She had been doing well until May 2012 when she was found to have a calcium level of 13.5 mg/dL (normal range: 8-10.5 mg/dL) with normal albumin during routine follow-up. She reported fatigue, nausea, and diarrhea, but no vomiting, abdominal pain or confusion. Workup revealed intact parathyroid hormone (PTH) level of 7 pg/mL (normal range: 11-80 pg/mL), PTH-related protein (PTH-rP) level of 23 pg/mL (normal range: 14-27 pg/mL), 25-hydroxyvitamin D level of 36 ng/mL (normal range: 30-100 ng/mL), but elevated 1,25-dihydroxyvitamin D level of 143 pg/mL (normal range: 18-72 pg/mL). Her calcium and vitamin D supplements were discontinued. She was given aggressive hydration and pamidronate with decreasing calcium level to 9.8 mg/dL. An MRCP showed mild interval increase in size of the innumerable liver metastases. Her hypercalcemia was postulated to be secondary to her underlying malignancy causing endogenous production of 1,25-dihydroxyvitamin D. Therefore, sunitinib was added to monthly octreotide in an attempt to slow progression, but her calcium levels still fluctuated between 10.3 and 13 mg/dL requiring intermittent hydration with diuresis, and monthly bisphosphonates. Denosumab and calcitonin were also used with suboptimal response.
In October 2012, the patient was admitted for acute kidney injury and was found to have acute tubular necrosis likely from overdiuresis. Her creatinine improved from 2.3 mg/dL (normal range: 0.5-1.1 mg/dL) to 1.2 mg/dL with hydration. Sunitinib was discontinued given concern for its association with increased serum creatinine. The combination of capecitabine (1,000 mg twice daily for 2 weeks followed by a 2-week break) and temozolomide (150 mg/m2 daily for the last 5 days of the capecitabine treatment) was initiated. Since then, she has been maintained on this regimen as well as octreotide every 4 weeks. Her calcium and 1,25-dihydroxyvitamin D levels normalized in January and July 2013 respectively. Her chromogranin A level decreased from a peak level of 196 ng/mL (range, 1.9-15 ng/mL) in October 2012 to 20 ng/mL in December 2013. The last MRCP in May 2014 showed decrease in disease burden within the liver and the pancreas (Figure 2A-C).
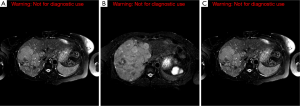
Discussion
Pancreatic neuroendocrine tumors are relatively rare with an annual incidence of 1.8 per 1 million in women and 2.6 in men according to the SEER database. Approximately 40% to 91% of pancreatic neuroendocrine tumors are nonfunctional (1). Our patient has a nonfunctional tumor as her gastrin, glucagon, vasoactive intestinal peptide levels were all normal and she had no fasting or nocturnal hypoglycemia.
Once distant metastases are discovered, if complete resection is not possible, the management is largely based on symptoms, tumor burden, and the tempo of the disease progression. The use of somatostatin analogs can be considered for asymptomatic patients who have positive octreotide scintography. The PROMID trial reported a significant longer time to tumor progression (14.3 vs. 6 months, P=0.000072) in the octreotide LAR arm compared with placebo in patients with both functional and nonfunctional metastatic midgut neuroendocrine tumors (2). CLARINET is a large phase III trial that randomized 204 patients with non-functional gastroenteropancreatic neuroendocrine tumors to either lanreotide or placebo. Progression-free survival (PFS), the trial’s primary end point, was significantly better with lanreotide (not reached vs. 18 months, P=0.0002) (3). Prior to the initiation of octreotide, our patient’s scan showed multiple foci of radiotracer uptake throughout the liver, supporting this treatment option.
Sunitinib was compared to placebo in patients with advanced, well-differentiated pancreatic neuroendocrine tumors. The daily dose of 37.5 mg was associated with improved PFS (11.4 vs. 5.5 months, P<0.001). The objective response with sunitinib was 9.3% as compared to 0% with placebo (4). Unfortunately, our patient was unable to tolerate this targeted therapy.
Temozolimide-base regimens have been studied recently in metastatic pancreatic neuroendocrine tumors. A retrospective study of 30 patients treated with a combination of capecitabine and temozolimide as first-line chemotherapy showed an objective radiographic response of 70% with median PFS of 18 months and survival rate of 92% at 2 years (5). Another retrospective analysis using the same regimen in metastatic, well-differentiated neuroendocrine cancers reported a total response rate of 61%. Among 18 patients studied, 1 patient with midgut carcinoid achieved a complete pathological response after resection (6). A prospective phase II study of capecitabine and temozolomide in 28 patients with metastatic, well-to-moderately differentiated neuroendocrine tumors was presented at the 2014 Gastrointestinal Cancers Symposium. Patients either progressed on long-acting octreotide or had negative octreotide scans. Overall response rate was 43% including 11% complete response, and stable disease rate was 54%, with clinical benefit in 97% of patients. The ongoing median PFS has reached 22 months. Twelve out of 28 patients had died at the time of interim analysis, yielding a median overall survival of more than 29 months. For 11 patients with pancreatic neuroendocrine tumors, the overall response rate was 45% with median PFS of more than 18.2 months (7).
Malignant hypercalcemia occurs in about 20-30% of patients with cancer, both solid tumors and hematologic malignancies (8). Among many mechanisms of action, a PTH-independent extrarenal production of 1,25-dihydoxyvitamin D from 25-hydroxyvitamin D contributes to almost all cases of hypercalcemia in lymphomas (9). In normal individuals, PTH regulates 1-hydroxylase in the kidney which converts 25-hydroxyvitamin D to 1,25-dihydroxyvitamin D. In the setting of hypercalcemia, the release of PTH will be suppressed, resulting in decreased production of 1,25-dihydroxyvitamin D. In patients with lymphomas, elevated 1,25-dihydroxyvitamin D production in a PTH-independent manner induces increased intestinal absorption of calcium and bone reabsorption to a lesser extent.
PTH-rP shares certain homology with PTH in that the first 13 amino acids are almost identical (10). As a result, it functions similarly to PTH by increasing bone and distal tubular reabsorption of calcium. However, the increased intestinal absorption of calcium does not occur with PTH-rP because it is less likely to stimulate 1,25-dihydroxyvitamin D production. PTH-rP is a gene product normally expressed in neuroendocrine tissues. The secretion of PTH-rP is the most common cause of malignant hypercalcemia and has been reported to be the etiology of hypercalcemia associated with neuroendocrine tumors (11-13). Our patient had a normal PTH-rP level. The low intact PTH level reflected physiologic suppression by hypercalcemia. She had a normal 25-hydroxyvitamin D level but 1,25-dihydoxyvitamin D was elevated. We therefore postulate that her hypercalcemia was a result of secretion of 1,25-dihydroxyvitamin D by the underlying metastatic pancreatic neuroendocrine tumor, a mechanism only commonly seen in lymphomas. This was further supported by the fact that the successful control of her disease with capecitabine and temozolomide led to the alleviation of this paraneoplastic syndrome.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Halfdanarson TR, Rabe KG, Rubin J, et al. Pancreatic neuroendocrine tumors (PNETs): incidence, prognosis and recent trend toward improved survival. Ann Oncol 2008;19:1727-33. [PubMed]
- Rinke A, Müller HH, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol 2009;27:4656-63. [PubMed]
- Delavault P, Caplin M, Liyange N, et al. The CLARINET study: assessing the effect of lanreotide autogel on tumor progression-free survival in patients with nonfunctioning gastroenteropancreatic neuroendocrine tumors. J Clin Oncol 2012;30:abstr TPS4153.
- Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med 2011;364:501-13. [PubMed]
- Strosberg JR, Fine RL, Choi J, et al. First-line chemotherapy with capecitabine and temozolomide in patients with metastatic pancreatic endocrine carcinomas. Cancer 2011;117:268-75. [PubMed]
- Fine RL, Gulati AP, Krantz BA, et al. Capecitabine and temozolomide (CAPTEM) for metastatic, well-differentiated neuroendocrine cancers: The Pancreas Center at Columbia University experience. Cancer Chemother Pharmacol 2013;71:663-70. [PubMed]
- Fine RL, Gulati AP, Tsushima D, et al. Prospective phase II study of capecitabine and temozolomide (CAPTEM) for progressive, moderately, and well-differentiated neuroendocrine tumors. J Clin Oncol 2014;32:abstr 179.
- Stewart AF. Clinical practice. Hypercalcemia associated with cancer. N Engl J Med 2005;352:373-9. [PubMed]
- Seymour JF, Gagel RF. Calcitriol: the major humoral mediator of hypercalcemia in Hodgkin’s disease and non-Hodgkin’s lymphomas. Blood 1993;82:1383-94. [PubMed]
- Burtis WJ, Brady TG, Orloff JJ, et al. Immunochemical characterization of circulating parathyroid hormone-related protein in patients with humoral hypercalcemia of cancer. N Engl J Med 1990;322:1106-12. [PubMed]
- Papazachariou IM, Virlos IT, Williamson RC. Parathyroid hormone-related peptide in pancreatic neuroendocrine tumours associated with hypercalcaemia. HPB (Oxford) 2001;3:221-5. [PubMed]
- Abraham P, Ralston SH, Hewison M, et al. Presentation of a PTHrP-secreting pancreatic neuroendocrine tumour, with hypercalcaemic crisis, pre-eclampsia, and renal failure. Postgrad Med J 2002;78:752-3. [PubMed]
- Kanakis G, Kaltsas G, Granberg D, et al. Unusual complication of a pancreatic neuroendocrine tumor presenting with malignant hypercalcemia. J Clin Endocrinol Metab 2012;97:E627-31. [PubMed]


