Centers with more therapeutic modalities are associated with improved outcomes for patients with hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common malignancy worldwide and is the third most common cause of cancer death worldwide. Management of HCC has many different treatment modalities (1). While surgery and transplantation are potential curative options, the majority of patients are not candidates for these therapies (2). For patients with unresectable disease, locoregional therapies include ablation, arterially directed therapies such as transarterial chemoembolization (TACE) or radioembolization, or stereotactic body radiation therapy (SBRT). Systemic therapy options include newer kinase inhibitors, such as, sorafenib, which has provided marginal increases in life span for advanced HCC patients (3). Chemotherapy options such as gemcitabine and oxaliplatin have demonstrated efficacy with overall disease control rates of 66% and 8.5% of the patients were subsequently eligible for curative-intent therapies after downstaging (4). More recently, immunotherapy using checkpoint blockade has demonstrated promise for the management of advanced HCC (5).
It has been well documented that treatment at high-volume facilities is associated with improved survival across many different disease sites (6-10). However, it is unknown if sites that offer multiple modalities of treatment, regardless of site volume, have improved overall survival (OS). We hypothesized that access to these multi-modality treatments for patients with HCC could result in improvement in OS. We conducted a nationwide cancer database analysis to assess if patients treated at facilities with more treatment modalities for HCC have improved OS.
Methods
Data source and study population
The study population used for this analysis was derived from the National Cancer Database (NCDB), which is a joint program of the Commission on Cancer and the American Cancer Society. The Commission on Cancer’s NCDB and the hospitals participating in the NCDB are the source of the deidentified data used in this study. It is estimated that the NCDB captures about 70% of newly diagnosed cancer cases in the United States (11). Patients were included in this analysis if they were diagnosed with non-metastatic HCC from 2004 to 2014.
Primary endpoint and covariates
The primary endpoint is the effect of facility treatment modality number on OS. The treatment modalities assessed were surgical resection, transplantation, ablation, radioembolization, SBRT, single-agent chemotherapy, and multi-agent chemotherapy. A facility was classified as using a particular modality if any patient treated at that site had been treated with that modality. The total number of modalities used for each facility was called the final treatment modality number. For illustrative purposes, facilities were dichotomized at the median as using ≤4 vs. ≥5 of the listed treatment modalities. Those using four or fewer modalities were classified as few-modality facilities and those using five or more modalities were classified as multi-modality facilities. Sensitivity analyses were also performed for cut points at two, three, five, and six modalities.
Other covariates included in the analysis were age, sex, race, insurance status (government vs. non-government), household income of patient’s zip code, education level of patient’s zip code, Charlson-Deyo comorbidity score (0 vs. 1 or 2), year of diagnosis, type of facility (academic vs. non-academic), geographical location of the facility, facility volume, clinical T stage, clinical N stage, tumor size, multiple vs. single tumor, presence of fibrosis, creatinine, bilirubin, and international normalized ratio (INR).
Statistical analysis
Descriptive statistics were used to summarize the baseline characteristics. Categorical variables were compared using the chi-squared test and continuous variables were assessed using the Student t-test or Mann-Whitney U test as appropriate. Facility treatment modality number was used as a dichotomous variable. Years of follow-up served as the time scale and were calculated beginning with the date of diagnosis to either final follow-up date or death, whichever occurred earliest. Kaplan-Meier survival curves were fit to visualize the distributions of time to death. The statistical significance between few-modality vs. multi-modality facilities was evaluated using the log rank test. Cox proportional hazard models were used to examine the multivariate association between facility modality number and OS. The proportional hazards assumption was tested using sums of weighted residuals.
To adjust for nonrandom patient assignment to facilities that may have been influenced by patient, disease, and/or geographically related characteristics potentially confounding survival estimates, we performed a propensity score analysis. Propensity scores were generated using multivariate logistic regression with the dichotomized variable facility modality number as the intervention of interest, and then subsequently compared the matched samples with OS as the outcome of interest. A 1:1 propensity score matching was used to match patients who were treated at a few vs. multi-modality facility, not allowing replacement and using a caliper width equal to 1.05 (0.2 of the standard deviation of the logit of the propensity score) (12). In the survival analysis, patients were propensity matched based on the following variables: facility volume, tumor size, single vs. multiple tumors, invasion into a major vessel, T stage, age, gender, year of diagnosis, race, insurance status, academic vs. community facility, location, Charlson-Deyo comorbidity score, income, education, level of fibrosis, creatinine, bilirubin, and INR. Kaplan-Meier curves for OS were generated for patients who were treated at a few vs. multi-modality facility.
Finally, sensitivity analyses were conducted changing the cut point from four to two, three, five, and six. Additional sensitivity analyses were conducted by removing the patients who had received rare treatment modalities. These modalities were defined as treatments which less than ten percent of the population received. These treatments were SBRT (0.7%), radioembolization (1.6%), transplant (7.0%), and ablation (7.3%). To determine this effect on different stages, stratified analyses based on stage were conducted. All analyses were conducted in Stata, version 12 (College Station, Texas, USA).
Results
Baseline characteristics stratified by few vs. multi-modality facility
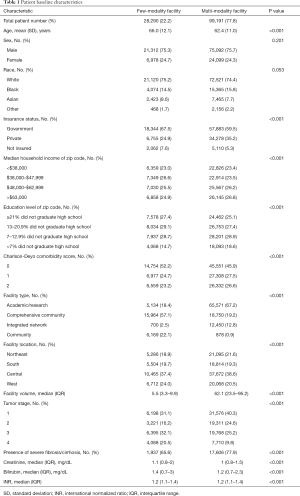
Baseline characteristics are summarized in Table 1. There was a total of 1,229 facilities. Of these, 829 (67.5%) used four or fewer treatment modalities and 400 (32.5%) used five or more treatment modalities. The median follow-up time for the entire population was 10.1 months (IQR: 2.6–28.2 months). There were 82,034 (72.9%) deaths in the population. In brief, there were 28,290 (22.2%) patients who were treated at few-modality facilities and 99,191 (77.8%) patients treated at multi-modality facilities. A much larger percentage of patients in the multi-modality facility group (67.2%) were treated at academic/research centers compared to 18.4% of patients in the few-modality facility group. Additionally, the median patient volume in the multi-modality facility group was 62.1 and 5.5 in the few-modality group. There was correlation (r=0.66, P<0.001) between facility volume and facility modality number.
Full table
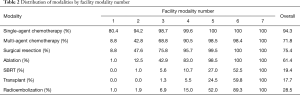
Table 2 shows the distribution of modality type by facility modality number. Single agent chemotherapy is the most commonly used treatment modality in the initial management of HCC patients and is the primary modality used in few-modality facilities. The next most common modalities used in the initial management of patients are multi-agent chemotherapy, surgical resection, and ablation. SBRT, transplant, and radioembolization are more rarely used in few-modality facilities. As facilities use more modalities, SBRT and transplant remain the least commonly used modalities.
Full table
Association between facility modality number and OS
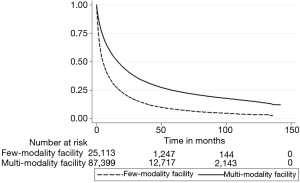
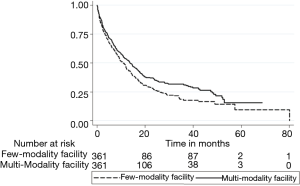
The Kaplan-Meier curve for Figure 1 illustrates the unadjusted relationship between facility modality number and OS (log-rank P<0.001). Cox proportional hazards models adjusting for age, sex, race, insurance status, household income of patient’s zip code, education level of patient’s zip code, Charlson-Deyo comorbidity score, year of diagnosis, type of facility, geographical location of the facility, facility volume, clinical T stage, clinical N stage, tumor size, multiple vs. single tumor, presence of fibrosis, creatinine, bilirubin, and INR can be seen in Table 3. Propensity matched analyses found similar results. The Kaplan-Meier curve (Figure 2) illustrates the propensity matched relationship between facility modality number and OS (log-rank P=0.032). In summary, increased facility modality usage was associated with a better OS [hazard ratio (HR) =0.60, 95% confidence interval (CI): 0.52–0.70, P<0.001] after adjusting for the above variables, including facility volume, age, race, insurance status, income, Charlson-Deyo comorbidity score, facility location, T stage, N stage, tumor size, presence of fibrosis, bilirubin, and INR were also significant predictors of OS.
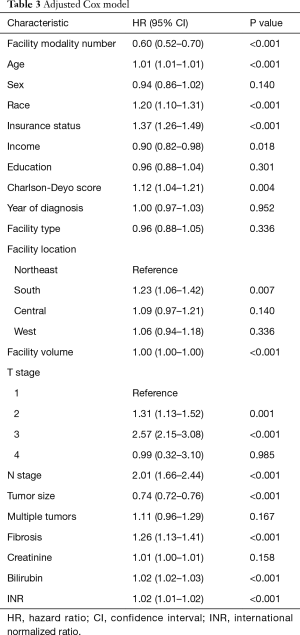
Full table
Sensitivity analyses were also performed. Varying the cut point from two to six showed that the adjusted HRs all remained significant [ranging from 0.36 (95% CI: 0.25–0.50, P<0.001) for a cut off of two to 0.89 (95% CI: 0.81–0.99, P=0.026) for a cut off of six] (Table 4). Less frequently used treatments were multi-agent chemotherapy (9.5%), ablation (8.2%), transplant (7.8%), radioembolization (1.6%), and SBRT (0.7%). Excluding patients who received these treatments in their initial management showed that treatment at a multi-modality facility continued to provide a survival advantage (adjusted HR =0.66, 95% CI: 0.56–0.77, P<0.001).
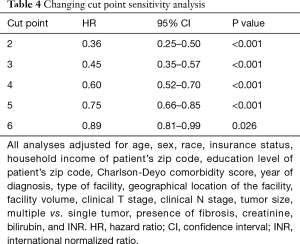
Full table
To determine if the benefit of higher modality number persisted across all stages, we conducted stratified analyses based on stage. For patients with stage I and II, facility modality number remains a predictor of OS in adjusted analyses (stage I: HR =0.48, 95% CI: 0.38–0.60, P<0.001; stage II: HR =0.64, 95% CI: 0.48–0.85, P=0.002). Facility modality number is not a significant predictor of OS for stage III (HR =0.83, 95% CI: 0.58–1.18, P=0.29) or stage IVa (HR =0.65, 95% CI: 0.34–1.27, P=0.21).
Discussion
To our knowledge, this is the first study to show that facilities that provide more treatment modalities have better OS after controlling for facility treatment volume. Several studies have shown that facilities with more treatment volume have better OS (6-10), but none have examined this relationship in HCC.
A possible reason for the effect seen is that patients being treated at centers with multi-modality therapy have access to the additional modalities once their disease progresses after initial management. This is consistent with the available data demonstrating that addition of locoregional therapies (13,14) or sorafenib (15,16) can improve survival when compared to best supportive care.
Another possibility is that increasing number of modalities maybe a proxy for quality of care delivered at these facilities (17). We tried to control for this in these analyses by adding facility volume and facility type to the model. Although we may not be able to completely control for this as there was correlation between facility volume and facility modality number, it is interesting to note that the HR for facility volume was 1.00 and facility type was not a significant predictor for OS. This shows that after accounting for facility modality number, facility volume and facility type are not significant predictors of OS. It is possible that facility modality number is a better proxy for the quality of care than facility volume (17). It is possible that facilities with more modalities available also have more collaborative tumor boards where patients are able to receive treatments that are better suited for their particular situation.
A possible confounder between facility modality number and OS is liver function. It is unclear which group has worse liver function. The multi-modality group had a higher percentage of patients with severe fibrosis/cirrhosis (78.0% vs. 66.2%). The few-modality group had slightly worse bilirubin (median of 1.4 vs. 1.3, P<0.001). While there was a statistically significant difference between the groups in bilirubin, INR and creatinine, the medians and IQRs are similar and there may be no clinically significant difference between the two groups. However, the few-modality group had a larger percentage of patients with stage III and IVa disease. To account for this, we ran the adjusted analysis stratifying patients by stage which showed that higher facility modality number was a predictor of OS for stage I and stage II patients but not stage III or IV. This is reassuring that the finding of improved OS remained true for patients with curable disease. It is possible that facility modality number is more important for curable disease because the optimal treatment has the potential to cure the disease and therefore would have a greater effect on increasing OS.
This study has several limitations. Selection bias cannot be completely ruled out where patients presenting at multi-modality centers maybe different than patients presenting to fewer modality centers. We tried to control for this in our multivariate analysis and by running an additional analysis where patients treated by excluding patients who were treated by the less frequently used treatment modalities. Another limitation of this analysis is that the data is its retrospective and only cases treated at COC accredited programs were reported. The NCDB did not have information related to cancer specific mortality and the Charlson-Deyo comorbidity score was the only available measure of comorbidity. The NCDB also does not provide separate data for TACE, which is why it could not be evaluated as a separate modality. It is possible that some of those patients were included in the radioembolization category or in the single-agent chemotherapy category. Similarly, it is impossible to know what proportion of the patients treated with single-agent chemotherapy were treated with sorafenib.
Conclusions
This study is the first to report to our knowledge that multi-modality facilities have better OS than facilities that use fewer modalities in patients with HCC. This remained true after controlling for facility volume and in sensitivity analysis after removing patients who received the less commonly used treatment modalities.
Acknowledgements
This work was supported by Department of Radiation Oncology, Montefiore Medical Center.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study only used a de-identified national database and was exempt from institutional review board (IRB) review.
References
- Waghray A, Murali AR, Menon KN. Hepatocellular carcinoma: From diagnosis to treatment. World J Hepatol 2015;7:1020-9. [Crossref] [PubMed]
- Eggert T, Greten TF. Current Standard and Future Perspectives in Non-Surgical Therapy for Hepatocellular Carcinoma. Digestion 2017;96:1-4. [Crossref] [PubMed]
- Shah C, Mramba LK, Bishnoi R, et al. Survival differences among patients with hepatocellular carcinoma based on the stage of disease and therapy received: pre and post sorafenib era. J Gastrointest Oncol 2017;8:789-98. [Crossref] [PubMed]
- Zaanan A, Williet N, Hebbar M, et al. Gemcitabine plus oxaliplatin in advanced hepatocellular carcinoma: a large multicenter AGEO study. J Hepatol 2013;58:81-8. [Crossref] [PubMed]
- El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017;389:2492-502. [Crossref] [PubMed]
- Liu CJ, Chou YJ, Teng CJ, et al. Association of surgeon volume and hospital volume with the outcome of patients receiving definitive surgery for colorectal cancer: A nationwide population-based study. Cancer 2015;121:2782-90. [Crossref] [PubMed]
- Luchtenborg M, Riaz SP, Coupland VH, et al. High procedure volume is strongly associated with improved survival after lung cancer surgery. J Clin Oncol 2013;31:3141-6. [Crossref] [PubMed]
- Pecorelli N, Balzano G, Capretti G, et al. Effect of surgeon volume on outcome following pancreaticoduodenectomy in a high-volume hospital. J Gastrointest Surg 2012;16:518-23. [Crossref] [PubMed]
- Reames BN, Ghaferi AA, Birkmeyer JD, et al. Hospital volume and operative mortality in the modern era. Ann Surg 2014;260:244-51. [Crossref] [PubMed]
- Yoshida EJ, Luu M, David JM, et al. Facility Volume and Survival in Nasopharyngeal Carcinoma. Int J Radiat Oncol Biol Phys 2018;100:408-17. [Crossref] [PubMed]
- Mohanty S, Bilimoria KY. Comparing national cancer registries: The National Cancer Data Base (NCDB) and the Surveillance, Epidemiology, and End Results (SEER) program. J Surg Oncol 2014;109:629-30. [Crossref] [PubMed]
- Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat 2011;10:150-61. [Crossref] [PubMed]
- Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 2002;35:1164-71. [Crossref] [PubMed]
- Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet 2002;359:1734-9. [Crossref] [PubMed]
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. [Crossref] [PubMed]
- Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009;10:25-34. [Crossref] [PubMed]
- Wuthrick EJ, Zhang Q, Machtay M, et al. Institutional clinical trial accrual volume and survival of patients with head and neck cancer. J Clin Oncol 2015;33:156-64. [Crossref] [PubMed]