Management of gastrointestinal carcinoid tumours - 10 years experience at a district general hospital
Introduction
Carcinoids are neuroendocrine tumours (NETs) arising from the diverse group of enterochromaffin cells. The common sites of carcinoid tumours include appendix, rectum, ileum, lung, bronchi and stomach (1-3). The overall incidence of carcinoid tumours has been steadily increasing and they are considered to be more aggressive with a poorer prognosis than previously thought (4-6). Gastrointestinal carcinoid tumours (GICTs) can be classified as foregut, midgut and hindgut carcinoids depending on the site of their origin. Diagnosis of GICTs can be challenging as most often they are asymptomatic and have advanced disease by the time of clinical presentation. The diagnosis of GICTs is often not made until after operative exploration for acute presentation with bowel obstruction, perforation or gastrointestinal bleeding. Although more recently staging systems for GICTs have been published, the long term prognosis is still not clear (7). Until recently there were noclear guidelines regarding diagnosis, referral, management and follow up of patients with GICTs (8). Most published studies on GICTs originate either from large tertiary centres or are based on pooled data from multicentre studies. The current study was done to review the literature on the management of patients with GICTs and to compare our experience in managing these patients at a district general hospital catering to a stable urban population within the National Health Service (NHS) over the last decade.
Materials and methods
This single centre, retrospective study was under taken at the South Tyneside District Hospital (STDH), South Shields, United Kingdom, between the period January 1999 to January 2009. The study was approved by the Trust audit and research committee. The hospital histopathology database was searched using the keywords 'carcinoid' and 'neuroendocrine tumour' to obtain a list of patients eligible for inclusion in the study. Only those patients with carcinoids arising from the gastrointestinal tract were included in the study. Using a pre-designed proforma, data including demographic information, presenting complaints, diagnostic methods, surgical procedures, histopathology and follow-up were extracted from the hospital case records and where necessary from contact with the patient's general practitioner. The data was transferred onto Microsoft Excelspreadsheet (Microsoft Corporation, Redmond, Washington, USA) and analysed using the statistical program SPSS version 15 (SPSS, Chicago, Illinois, USA).
An important part of the study was to critically review the current evidence on the management of GICTs and thus, a systematic literature search of PubMed, Ovid and Cochrane was performed for keywords "gastrointestinal carcinoids and gastrointestinal neuro-endocrine tumours". Relevant publications were reviewed and compared with our results at STDH in order to generate conclusions and recommendations for the management of GICTs within the confines of small district hospitals.
Results
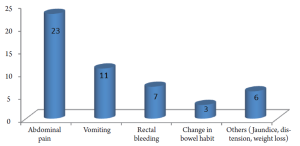
At STDH which is a small hospital providing healthcare services to a population of about 160,000, a total of 35 patients (17 males, 18 females; mean age 62±15.7 years, ranging from 20 to 92 years) were identified for inclusion in this study. This gives an estimated annual incidence of around 2.2 per 100,000 population. Patients presented with a wide spectrum of symptoms but by no means specific to carcinoid tumours. Figure 1 summarises the symptomatology in patients from the current study; the most frequently encountered symptoms being abdominal pain (66%), vomiting (31%) followed by rectal bleeding (20%). None of the patients in this series had symptoms of carcinoid syndrome.
Whilst pre-operative diagnosis of carcinoid tumour was confirmed following endoscopic biopsy of suspicious lesions in 13 (37%) patients; the remaining 22 (63%) patients had their definitive diagnosis established by immunohistopathology of the resected specimens following surgery. A total of 24 (69%) patients had a CT scan of abdomen, of which 4 (11%) had mesenteric lymph node mass and 6 (17%) had evidence of distant metastases in liver and/or lung. CT scan of one patient with midgut carcinoid demonstrating a circumscribed mesenteric mass with associated radiating mesenteric stranding is shown in Figure 2; this finding is considered to be rare but pathognomonic of small bowel carcinoid (3). Urinary 5-hydroxyindolacetic acid (5-HIAA) levels were checked in 6 (17%) patients and this was elevated in 2 patients (9); both patients had mutiple liver metastases.
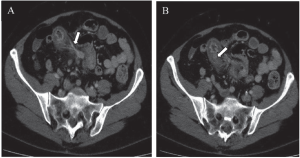
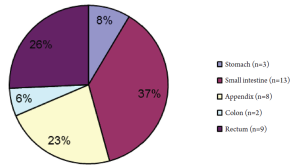
Figure 3 summarises the distribution of the GICTs in the current study and the majority of these tumours 21 (60%) were midgut carcinoid tumours. Of note, 16 (76%) of these patients presented acutely with abdominal pain and/or small bowel obstruction needing emergency surgery. Details of the surgical treatment of all patients are shown in Table 1. The type and the extent of the surgery varied with the site of the primary, presence of advanced disease and patient's performance status. Twenty seven (77%) patients had localised disease and were operated with a curative intent. Of the remaining 8 (23%) patients, 6 (17%) had extensive mesenteric lymph node involvement and 2 (7%) had distant visceral metastasis; surgery being either diagnostic biopsy only (n=4) or palliative resection (n=4). There were no peri-operative deaths but 2 patients who had emergency laparotomy for small bowel obstruction secondary to ileal carcinoids had to be re-operated for anastomotic leaks.

Full table
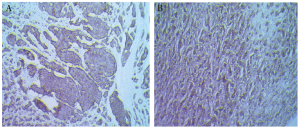
The size of the primary tumour on histology of the resected specimens ranged from 0.8 to 3.6 cm with a mean size of 2±0.9 cm. Immunohistochemistry was typical for carcinoids across all the tumours showing positive staining with Synaptophysin and Chromogranin A (Figure 4). 31 out of the 35 (89%) GICTs were well differentiated neuro-endocrine carcinomas with < 2 mitoses per high power field.
Whilst there was no fixed protocol for follow up schedule and investigations, all cases were discussed at the local GI MDTs. Referral to regional neuroendocrine multidisciplinary team (NET-MDT) services started in 2006 and of the 18 patients who were diagnosed with GICTs post 2006, 10 (56%) patients were referred to this service. In the current study, after a median follow up of 24 months (range 2-96 months), 22 patients (63%) were alive and disease free and 4 patients (11%) were alive with disease. Seven patients (20%) have died with disease and 2 patients were lost to follow up.
Discussion
GICTs account for approximately 75% of all neuro-endocrine tumours and according to a recent large population based survey there has been a significant increase in the annual age adjusted incidence rates of carcinoids tumours (from 1.09/100,000 in 1973 to 5.25/100,000 in 2004) (6). Based on their embryonic origin, GICTs are classified as foregut, midgut and hindgut carcinoids. They are slow growing lesions and as a result, patients usually complain of a wide variety of non-specific abdominal pains/symptoms which eventually progress to episodes of small bowel obstruction (10) or rarely gastrointestinal bleeding. Furthermore, in a significant proportion of these patients, the diagnosis is not revealed until after emergency surgery. Table 2 summarises the distribution, characteristics and clinical manifestations of GICTs from the pooled data of the large epidemiological studies of Modlin et al. (4) and Robertson et al. (5); it is to be noted that the findings in the current study were similar (see Figure 3).

Full table
Gastric carcinoids account for less than 1% of all gastric neoplasms but up to 6% of GICTs (1,11). Depending on the clinical and histological features, they are classified into three sub-groups: those associated with chronic atrophic gastritis type A (CAG-A), those associated with Zollinger-Ellison syndrome (ZES) and sporadic gastric carcinoids. CAG-A associated carcinoids are usually less than 1 cm, multifocal and predominantly located in the body and fundus; they follow an indolent course with less than 10% associated with distant metastasis (12-14). In contrast, the sporadic types (15-20% of all gastric carcinoids) are usually solitary, measure more than 1 cm and display a more aggressive clinical course with the majority associated with distal disease at presentation (12). About 5-10% of gastric carcinoids are associated with ZES and almost exclusively occur in patients with multiple endocrine neoplasia type 1; the clinical features and prognosis are similar to CAG-A associated carcinoids.
Midgut carcinoids (MCs) account for a third of all GICTs and 25 per cent of all small bowel tumours. They are more common in the 6th and 7th decades, predominant in males and represent the most common cause of the carcinoid syndrome (4). They are usually multicentric, located in the distal ileum and thought to arise from serotonin producing intra-epithelial endocrine cells. MCs have significant malignant potential with 50% to 60% of patients having metastatic disease at time of diagnosis (4). The patients usually have a long history of abdominal discomfort/pain which eventually require admission because of obstruction, perforation or gastrointestinal bleeding (3,15).
The primary lesion in MCs is usually a small (<1 cm), flat and fibrotic tumour in the submucosal plane of the ileum and is frequently not diagnosed until surgical exploration. Other operative findings usually include enlarged lymph nodes with associated adjacent mesenteric fibrosis (3) leading to kinking of the bowel and thus obstruction (10,16). This extensive mesenteric stranding and fibrosis is probably secondary to the release of serotonin and growth factors (from tumour cells) and can also lead to the encasement of mesenteric vessels leading to ischemia of the bowel (10).
Appendiceal carcinoids are the most common malignant tumours of the appendix and are diagnosed incidentally in 0.3-0.9 per cent of patients undergoing appendicectomy (17). They are usually diagnosed in the fourth and fifth decades of life (11). Appendiceal carcinoids are more common in women (11), usually located in the distal third of the appendix where they do not cause any obstruction and thus remain asymptomatic (18). Size of the tumour is considered to be of prognostic value with more than 95 per cent of appendiceal carcinoids being less than 2 cm and
rarely metastasising (19). In such patients, simple appendicectomy is curative whereas those whose tumours are greater than 2 cm, should in addition be treated with right hemicolectomy (18). Treatment for lesions between 1 and 2 cm is controversial and the decision for right hemicolectomy depends on factors like mesoappendiceal invasion, vascular invasion, mitotic activity, proliferation markers and patient risk factors (20,21). Goblet cell appendiceal carcinoids tend not to produce a grossly visible tumour mass but diffusely infiltrate the wall and have features of both carcinoid and adenocarcinoma (22,23). These patients should be offered hemicolectomy.
Colonic carcinoids account for about 12% of all carcinoid tumours but only 1% of colonic tumours. There is a slight female predominance and most patients commonly present in the seventh decade (24) with symptoms of pain, anorexia and weight loss (25); two thirds of colonic carcinoids being found on the right side with the majority in the caecum (25,26). Amongst all of the GICTs, colonic carcinoids are associated with a worse prognosis (27), with most of the patients presenting with advanced disease with an average tumour size of 5 cms and over two thirds having nodal and/or distant metastases (25,26). Colonic carcinoids are usually non-functional with less than 5 per cent containing cells producing serotonin and these patients can present with carcinoid syndrome (25,28,29). Clinically colonic carcinoids should be managed as colonic adenocarcinomas with radical colectomy and metastasectomy as appropriate (15,30). Also, if present, widespread metastatic disease should not preclude removal of the primary lesion (15).
Rectal carcinoids account for 2 per cent of rectal tumours and differ from other GICTs in that the neuroendocrine cells contain mostly glucagons and glicentin related peptides rather than serotonin (31). They are most common in the sixth decade of life (11), with patients presenting with pain, constipation and rectal bleeding. However, nearly half of the patients are asymptomatic and the lesions are found incidentally at routine endoscopy (32). The majority of these tumours are less than 1 cm in size and are best treated by local excision (32), whereas those lesions greater than 2 cm have traditionally been treated with anterior resection or abdomino-perineal resection. This practice has been recently questioned as there does not appear to be much survival advantage over and above that achieved by local excision (33,34).
The clinical course of patients with metastatic carcinoid tumour is highly variable, with some patients remaining symptom-free for years (19). Liver is the commonest site of distal spread from GICTs which also rarely metastasise to extra-abdominal organs including bone, lung, central nervous system, mediastinal and cervical lymph nodes. Carcinoid liver metastases tend to be hypervascular and thus appear isodense on conventional post-contrast CT scans (35,36). Resection of carcinoid liver metastasis is indicated in fit patients who do not have extra-hepatic metastatic disease, no tricuspid valve deficiency and have a resectable primary disease (37). Selective hepatic trans-catheter arterial embolization and trans-catheter arterial chemoembolisation can be used to treat liver metastasis in patients where major resections are not feasible. Radiofrequency ablation of liver metastases has been attempted in patients where arterial embolisation fails. Response rate of 80-95% have been reported following this treatment (38,39).
Carcinoid syndrome is typically seen in patients with liver/lung metastases with an overall incidence of 10% in GICTs but 20% in those with jejuno-ileal disease (2,4). Typical symptoms of carcinoid syndrome include flushing, diarrhoea, tachycardia, hypotension, bronchospasm and right heart failure; carcinoid heart disease is a late and potentially fatal complication presumed to be secondary to endocardial fibrosis of right side heart valves leading to pulmonary stenosis and tricuspid insufficiency. Whilst the exact chemicals responsible for the symptoms are yet to be clearly defined, the diarrhoea is probably secondary to excessive circulating levels of serotonin whereas bronchospasm is due to both serotonin and bradykinin. Prolonged high serum levels of serotonin is responsible for carcinoid heart disease (40) but valves of the left side of the heart are less affected because of the metabolism of serotonin within the lungs. Measurement of elevated levels of urinary 5-HIAA have been shown to be of value in predicting carcinoid syndrome (41). Somatostatin analogues are effective in controlling the symptoms and improve the quality of life in patients with carcinoid syndrome. They can be administered pre-operatively and continued for about 48 hours in the post-operative period to prevent a carcinoid crisis (37).
Diagnosis
Biochemical tests
Carcinoid tumour cells originate from neuroendocrine cells and are capable of synthesis, storage and release of serotonin, histamine, prostaglandin, kallikrenin, bradykinin, substance P, gastrin, corticotrophin and neuron specific enolase. The most abundant of these is serotonin (5-hydroxytryptamine) which after metabolising is converted to 5-hydroxyindolacetic acid (5-HIAA), Determination of raised 5-HIAA levels in 24-hr urine samples is routinely used for diagnosis of carcinoids but it is neither specific nor sensitive as it may not be elevated in some carcinoids whereas it can be elevated in conditions like tropical sprue, coeliac disease, Whipple's disease and small bowel obstruction (5). Serum chromogranin A levels have been shown to reflect tumour load and provide evidence of persistent or recurrent carcinoid disease and is a useful parameter to monitor disease spread and recurrence (42). Presence of carcinoembryonic antigen is a poor prognostic indicator and these tumours are often classified as adenocarcinoids and treated as adenocarcinomas rather than carcinoids (43,44).
Radiology
Plain abdominal films may show features consistent with obstruction (dilated bowel loops with thickened walls). CT rarely demonstrate the primary lesion but the delineation of a circumscribed mesenteric mass with associated radiating mesenteric stranding is considered to be pathognomonic of a GICT [as illustrated in one of our patients (Figures 2a and 2b)]. CT is also used to identify liver metastases but as these lesions are hypervascular (vide supra), a porto-venous phase scan is considered to be more sensitive in diagnosing smaller lesions (35,36). GICT cells are rich in somatostatin receptors which have a high affinity for Octreotide and as a result, Octreoscan (a radio-labelled Octreotide scintigraphy) is currently employed to detect metastases and recurrent disease with more than 90% sensitivity (45). Similarly, positron emission tomography (PET) with 5-hydroxytrytophan (serotonin precursor) labelled with C (5HTP-PET) has been shown to have high specificity and is now used to monitor the effects of therapy (46).
Histology
Depending on the histological appearances, mitotic and proliferation indices, GICTs are classified as well differentiated neuroendocrine tumours, well differentiated endocrine carcinomas, poorly differentiated endocrine carcinomas and mixed exocrine/endocrine tumours (47). Proliferation index is assessed using immunostaining with Ki67 antibody and is usually low (<2%) in classical MCs. Whilst 85% of all MCs and their metastases react to chromogranin A and synaptophysin (Figure 3) positive immunoreactivity to serotonin on the other hand, implies that the primary tumour originates from the midgut (2,48).
Treatment
Surgery continues to be the main modality of treatment for GICTs with a potential to cure in early stage disease and providing best palliation in those with advanced disease. Whilst the type and nature of surgery depends on the site and extent of the primary lesion, it is important to note that most patients with MCs are subjected to laparotomy without the awareness of a diagnosis of carcinoid tumour. Usually a wedge resection including the bowel segment containing the primary tumour and the involved lymph nodes are excised; this procedure is also indicated in those patients with synchronous liver metastases, as local disease if left untreated can lead to significant morbidity (2). Despite curative primary surgery, 80% of patients with MCs develop recurrence and these are usually evident after a median follow up of 5-10 years (3). The recurrent disease plus mesenteric fibrosis can manifest as chronic abdominal pain, intestinal obstruction and/or bowel ishaemia necessitating further surgical intervention (49,50) but earlier diagnosis of the recurrence can often be accomplished by serial estimation of serum chromogranin A levels (10).
Recently prophylactic surgery to remove mesenterico-intestinal tumour in asymptomatic patients has been advocated because patients who receive and survive medical treatment can still present with major intra-abdominal complications from the mesenteric disease (2). Pre-operative mapping of the extent of the disease within the mesentery and assessment of the involvement of the root of the major mesenteric vessels with dynamic CT scan is now considered mandatory in treatment planning. Tumour debulking in patients with advanced mesenteric metastases in the absence of liver metastases has been reported to achieve a 5-year survival of 91% (with a median survival of 12.4 years) (51). Operating on patients with carcinoid syndrome can induce carcinoid crisis (hyperthermia, shock, arrhythmia, excessive flush and bronchial spasm) and as a prophylaxis, it is important for these patients to be given intravenous octreotide (500 μg in 500 mL saline, 50 mL/hour) during surgery.
Liver metastases
The majority of carcinoid patients with liver metastases have multiple lesions widely spread in both lobes and need medical treatment including somatostatin and interferon, which are effective in symptom control as well as increasing the life expectancy (42). Less than 10% of patients have solitary or dominant lesions which are amenable for surgical resection (10). Other modalities used to treat liver metastases include radiofrequency ablation, liver embolisation, transplantation, radioactive labelled octreotide and meta-iodobenzylguanidine (MIBG) (2,42). Radiofrequency ablation which is being increasingly used, can induce necrosis of lesions up to 3-4 cm in size but, is not very effective for lesions close to major vessels. Significant and sustained symptom relief as well as reduction in tumour markers can be achieved if greater than 90% of tumour volume has been ablated or excised (52). Almost all patients who had curative liver resection will develop new metastases which typically show slow progression and are unusually tenacious; imaging even with specific methods such as octreotide scan and 5-HTP PET may fail to detect disease progression.
Medical treatment
Somatostatin is a peptide that inhibits the secretion of a number of hormones (growth hormone, insulin, glucagon and gastrin) (53) and a significant proportion of GICTs (>80%) have been shown to express somatostatin receptors on their cell surfaces. As a result, somatostatin analogues (Octreotide and Lanreotide) as well as interferons have been used to effectively palliate the symptoms of carcinoid syndrome in up to 70% of patients, to provide tumour reduction in around 5% of patients and to stabilise the disease (an average of three years) in approximately half of the patients (40,54,55). These analogues can be self-administered thrice daily (50-150 µg) through subcutaneous injections and furthermore, longer acting formulations with the convenience of once monthly injections are also available (54). The side effects of octreotide treatment are gallstone formation and pancreatic insufficiency whereas those patients receiving interferons report more adverse effects including flu-like symptoms, chronic fatigue and autoimmune reactions. Chemotherapy has yielded limited success in the treatment of GICTs and tend to be more effective in patients with aggressive variants (such as neuroendocrine carcinomas), in which a cisplatin/etoposide-based regime has been reported to achieve up to 60% response rate (56). External beam radiotherapy has been shown to have a role in the treatment of locally unresectable disease and in the effective palliation of bone and central nervous system metastases (57).
Role of multi-disciplinary team
Patients with carcinoid tumours require multiple modalities of treatment and a dedicated multi-disciplinary team (MDT) to co-ordinate the management and follow up is essential. In view of the relatively low incidence of these tumours and for optimum use of resources, these MDTs need to be centralised and based in regional tertiary centres (8). A neuro-endocrine tumour (NET) MDT should include gastroenterologists, surgeons, oncologists, endocrinologists, radiologists, nuclear medicine specialists histopathologists and an MDT co-ordinator; the team should meet weekly or fortnightly depending on the case load (8). In north-east England, NET-MDT service started in 1999 and is based at Freeman Hospital, Newcastle upon Tyne which is the regional tertiary centre. However, the utilisation of this service by the clinicians in peripheral hospitals in the region started much latter. In the current study GICT patients were referred to the regional NET MDT from 2006 onwards and in that period only 10 out of 18 patients (56%) with GICTs were referred to this MDT; details of radiology, surgery and histology were reviewed and further management and follow up plans made.
Prognosis and follow up
Prognosis of patients with GICTs is largely determined by age, race and sex of patients, site and size of the primary lesion, stage of the disease, histologic grade and extent of the disease (6). The incidence of nodal and distant metastasis is rare if the primary tumour size is less than 1 cm, however this increases significantly once the primary lesion is over 2 cm in size (58). As described above, patients with carcinoid tumours in appendix and rectum carry a better prognosis with a five year survival ranging from 62-100% depending on the size of the primary lesion. Patients with small intestinal and colonic carcinoids carry a poorer prognosis with a five year survival ranging from 33-75%. Large patient series from Sweden (1960-2000) and from USA (1973-1999) have reported an age adjusted 5-year survival rates of 67% for midgut carcinoids (4,59). In a series of over 300 patients median survival was 12.4 years and 5-year survival was 91% in the absence of liver metastasis and 50% in patients with inoperable liver metastases (51). Significant symptom relief and long disease free survival have been consistently been reported following liver surgery in patients with carcinoid syndrome (2,10,45). Five year survival of over 70% has been reported following radical curative liver resection but nearly all will eventually develop new metastases, often with slow progression. In fact, long term follow-up studies have identified the presence of liver metastases and carcinoid heart disease as the two most significant adverse prognostic indicators (51,59).
The current study is a small series of abdominal carcinoid tumours treated at a single institution but it does represent a modest experience of midgut carcinoids (n=21). Compared to larger published studies, the median follow up time was only 24 months with the longest follow up time being 8 years and this could be partly attributed to some patients being followed up in the regional tertiary centre following referral after initial treatment locally. As there is increasing evidence to suggest that recurrences can become clinically overt after a median of 5-10 years, clearly no concrete data about recurrence and survival can be inferred until the patients have completed a further prolonged follow up period. Furthermore, the study is also underpowered to provide information about differences in survival in those patients who were referred to the regional centre (post 2006) compared to those treated locally.
Conclusions
Currently there is still a lack of clear guidelines for referral and follow up of patients diagnosed incidentally with GICTs particularly within the setting of district general hospitals within the UK. With the recent published evidence about the staging, treatment options and prognosis of carcinoid tumours, this should be feasible and efforts should be made to align the delivery of care to these patients in tandem with the tertiary centres. The hope remains that better and / or modern treatment pathways for carcinoid tumours delivered in a regional setting would be reflected in a difference in survival. Hence, there is a need for more NET-MDTs nationwide in order to provide a co-ordinated approach in the management of this rare condition.
Acknowledgements
The authors will like to thank the help of Dr K. Jain, Consultant pathologist, South Tyneside General Hospital, South Shields for her help in searching the histopathology database for obtaining the list of patients eligible for inclusion in the study and also providing the photomicrographs of immune-histochemical staining.
Footnote
No potential conflict of interest.
References
- Godwin JD II. Carcinoid tumors. An analysis of 2,837 cases. Cancer 1975;36:560-569. [PubMed]
- Akerström G, Hellman P, Hessman O, Osmak L. Management of midgut carcinoids. J Surg Oncol 2005;89:161-169. [PubMed]
- Akerstorm G. Companion to specialist surgical practice: endocrine surgery, 2005.
- Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer 2003;97:934-959. [PubMed]
- Robertson RG, Geiger WJ, Davis NB. Carcinoid tumors. Am Fam Physician 2006;74:429-434. [PubMed]
- Yao JC, Hassan M, Phan A, et al. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008;26:3063-3072. [PubMed]
- Landry CS, Brock G, Scoggins CR, McMasters KM, Martin RC II. A proposed staging system for small bowel carcinoid tumors based on an analysis of 6,380 patients. Am J Surg 2008;196:896-903, discussion 903. [PubMed]
- Ramage JK, Davies AH, Ardill J, et al. Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours. Gut 2005;54:Siv1-Siv16.
- van der Horst-Schrivers AN, Post WJ, Kema IP, et al. Persistent low urinary excretion of 5-HIAA is a marker for favourable survival during follow-up in patients with disseminated midgut carcinoid tumours. Eur J Cancer 2007;43:2651-2657. [PubMed]
- Kerström G, Hellman P, Hessman O. Midgut carcinoid tumours: surgical treatment and prognosis. Best Pract Res Clin Gastroenterol 2005;19:717-728. [PubMed]
- Modlin IM, Sandor A. An analysis of 8305 cases of carcinoid tumors. Cancer 1997;79:813-829. [PubMed]
- Rindi G, Bordi C, Rappel S, La Rosa S, Stolte M, Solcia E. Gastric carcinoids and neuroendocrine carcinomas: pathogenesis, pathology, and behavior. World J Surg 1996;20:168-172. [PubMed]
- Rindi G, Luinetti O, Cornaggia M, Capella C, Solcia E. Three subtypes of gastric argyrophil carcinoid and the gastric neuroendocrine carcinoma: a clinicopathologic study. Gastroenterology 1993;104:994-1006. [PubMed]
- Gough DB, Thompson GB, Crotty TB, et al. Diverse clinical and pathologic features of gastric carcinoid and the relevance of hypergastrinemia. World J Surg 1994;18:473-479, discussion 479-480. [PubMed]
- Modlin IM, Kidd M, Latich I, Zikusoka MN, Shapiro MD. Current status of gastrointestinal carcinoids. Gastroenterology 2005;128:1717-1751. [PubMed]
- Moertel CG, Dockerty MB, Judd ES. Carcinoid tumors of the vermiform appendix. Cancer 1968;21:270-278. [PubMed]
- Goede AC, Caplin ME, Winslet MC. Carcinoid tumour of the appendix. Br J Surg 2003;90:1317-1322. [PubMed]
- Moertel CG, Weiland LH, Nagorney DM, Dockerty MB. Carcinoid tumor of the appendix: treatment and prognosis. N Engl J Med 1987;317:1699-1701. [PubMed]
- Kulke MH, Mayer RJ. Carcinoid tumors. N Engl J Med 1999;340:858-868. [PubMed]
- Syracuse DC, Perzin KH, Price JB, Wiedel PD, Mesa-Tejada R. Carcinoid tumors of the appendix. Mesoappendiceal extension and nodal metastases. Ann Surg 1979;190:58-63. [PubMed]
- Ponka JL. Carcinoid tumors of the appendix. Report of thirty-five cases. Am J Surg 1973;126:77-83. [PubMed]
- Carr NJ, Sobin LH. Neuroendocrine tumors of the appendix. Semin Diagn Pathol 2004;21:108-119. [PubMed]
- Stinner B, Rothmund M. Neuroendocrine tumours (carcinoids) of the appendix. Best Pract Res Clin Gastroenterol 2005;19:729-738. [PubMed]
- Spread C, Berkel H, Jewell L, Jenkins H, Yakimets W. Colon carcinoid tumors. A population-based study. Dis Colon Rectum 1994;37:482-491. [PubMed]
- Rosenberg JM, Welch JP. Carcinoid tumors of the colon. A study of 72 patients. Am J Surg 1985;149:775-779. [PubMed]
- Ballantyne GH, Savoca PE, Flannery JT, Ahlman MH, Modlin IM. Incidence and mortality of carcinoids of the colon. Data from the Connecticut Tumor Registry. Cancer 1992;69:2400-2405. [PubMed]
- Waisberg DR, Fava AS, Martins LC, Matos LL, Franco MI, Waisberg J. Colonic carcinoid tumors: a clinicopathologic study of 23 patients from a single institution. Arq Gastroenterol 2009;46:288-293. [PubMed]
- Cheng JY, Lin JC, Yu DS, Lee WH, Meng CL. Flow cytometric DNA analysis of colorectal carcinoid. Am J Surg 1994;168:29-32. [PubMed]
- Berardi RS. Carcinoid tumors of the colon (exclusive of the rectum): review of the literature. Dis Colon Rectum 1972;15:383-391. [PubMed]
- Lyda MH, Fenoglio-Preiser CM. Adenoma-carcinoid tumors of the colon. Arch Pathol Lab Med 1998;122:262-265. [PubMed]
- Capella C, Heitz PU, Höfler H, Solcia E, Klöppel G. Revised classification of neuroendocrine tumours of the lung, pancreas and gut. Virchows Arch 1995;425:547-560. [PubMed]
- Jetmore AB, Ray JE, Gathright JB Jr, McMullen KM, Hicks TC, Timmcke AE. Rectal carcinoids: the most frequent carcinoid tumor. Dis Colon Rectum 1992;35:717-725. [PubMed]
- Sauven P, Ridge JA, Quan SH, Sigurdson ER. Anorectal carcinoid tumors. Is aggressive surgery warranted? Ann Surg 1990;211:67-71. [PubMed]
- Koura AN, Giacco GG, Curley SA, Skibber JM, Feig BW, Ellis LM. Carcinoid tumors of the rectum: effect of size, histopathology, and surgical treatment on metastasis free survival. Cancer 1997;79:1294-1298. [PubMed]
- Woodard PK, Feldman JM, Paine SS, Baker ME. Midgut carcinoid tumors: CT findings and biochemical profiles. J Comput Assist Tomogr 1995;19:400-405. [PubMed]
- Sugimoto E, Lörelius LE, Eriksson B, Oberg K. Midgut carcinoid tumours. CT appearance. Acta Radiol 1995;36:367-371. [PubMed]
- Poncet G, Faucheron JL, Walter T. Recent trends in the treatment of well-differentiated endocrine carcinoma of the small bowel. World J Gastroenterol 2010;16:1696-1706. [PubMed]
- Siperstein AE, Rogers SJ, Hansen PD, Gitomirsky A. Laparoscopic thermal ablation of hepatic neuroendocrine tumor metastases. Surgery 1997;122:1147-1154, discussion 1154-1155. [PubMed]
- Wessels FJ, Schell SR. Radiofrequency ablation treatment of refractory carcinoid hepatic metastases. J Surg Res 2001;95:8-12. [PubMed]
- de Vries H, Verschueren RC, Willemse PH, Kema IP, de Vries EG. Diagnostic, surgical and medical aspect of the midgut carcinoids. Cancer Treat Rev 2002;28:11-25. [PubMed]
- Feldman JM, O'Dorisio TM. Role of neuropeptides and serotonin in the diagnosis of carcinoid tumors. Am J Med 1986;81:41-48. [PubMed]
- Öberg K. Carcinoid Tumors: Current Concepts in Diagnosis and Treatment. Oncologist 1998;3:339-345. [PubMed]
- Bishopric GA Jr, Ordóñez NG. Carcinoembryonic antigen in primary carcinoid tumors of the lung. Cancer 1986;58:1316-1320. [PubMed]
- Federspiel BH, Burke AP, Shekitka KM, Sobin LH. Carcinoembryonic antigen and carcinoids of the gastrointestinal tract. Mod Pathol 1990;3:586-590. [PubMed]
- Ganim RB, Norton JA. Recent advances in carcinoid pathogenesis, diagnosis and management. Surg Oncol 2000;9:173-179. [PubMed]
- Orlefors H, Sundin A, Ahlström H, et al. Positron emission tomography with 5-hydroxytryprophan in neuroendocrine tumors. J Clin Oncol 1998;16:2534-2541. [PubMed]
- Rindi G, Candusso ME, Solcia E. Molecular aspects of the endocrine tumours of the pancreas and the gastrointestinal tract. Ital J Gastroenterol Hepatol 1999;31:S135-S138. [PubMed]
- Van Eeden S, Quaedvlieg PF, Taal BG, Offerhaus GJ, Lamers CB, Van Velthuysen ML. Classification of low-grade neuroendocrine tumors of midgut and unknown origin. Hum Pathol 2002;33:1126-1132. [PubMed]
- Söreide JA, van Heerden JA, Thompson GB, Schleck C, Ilstrup DM, Churchward M. Gastrointestinal carcinoid tumors: long-term prognosis for surgically treated patients. World J Surg 2000;24:1431-1436. [PubMed]
- Gulec SA, Mountcastle TS, Frey D, et al. Cytoreductive surgery in patients with advanced-stage carcinoid tumors. Am Surg 2002;68:667-671, discussion 671-672. [PubMed]
- Hellman P, Lundström T, Ohrvall U, et al. Effect of surgery on the outcome of midgut carcinoid disease with lymph node and liver metastases. World J Surg 2002;26:991-997. [PubMed]
- Poncet G, Faucheron JL, Walter T. Recent trends in the treatment of well-differentiated endocrine carcinoma of the small bowel. World J Gastroenterol 2010;16:1696-1706. [PubMed]
- Somatostatin S. Somatostatin. N Engl J Med 1983;309:1495-1501. [PubMed]
- Oberg K. Carcinoid tumors: molecular genetics, tumor biology, and update of diagnosis and treatment. Curr Opin Oncol 2002;14:38-45. [PubMed]
- Bousquet C, Puente E, Buscail L, Vaysse N, Susini C. Antiproliferative effect of somatostatin and analogs. Chemotherapy 2001;47:30-39. [PubMed]
- Moertel CG, Kvols LK, O'Connell MJ, Rubin J. Treatment of neuroendocrine carcinomas with combined etoposide and cisplatin. Evidence of major therapeutic activity in the anaplastic variants of these neoplasms. Cancer 1991;68:227-232. [PubMed]
- Schupak KD, Wallner KE. The role of radiation therapy in the treatment of locally unresectable or metastatic carcinoid tumors. Int J Radiat Oncol Biol Phys 1991;20:489-495. [PubMed]
- Rorstad O. Prognostic indicators for carcinoid neuroendocrine tumors of the gastrointestinal tract. J Surg Oncol 2005;89:151-160. [PubMed]
- Zar N, Garmo H, Holmberg L, Rastad J, Hellman P. Long-term survival of patients with small intestinal carcinoid tumors. World J Surg 2004;28:1163-1168. [PubMed]