
Racial disparities in the incidence of colon cancer in patients with inflammatory bowel disease
Introduction
Inflammatory bowel disease (IBD), which includes Crohn’s disease and ulcerative colitis (UC), is a debilitating disease that affects 1.4 million people in the United States (US) (1,2). The incidence of IBD is strikingly high in North America, ranging from 2.2 to 19.2 cases per 100,000 person-years (3,4). There is a significant heritable component of this disease, with first degree relatives having approximately a 3–20 times increased likelihood of developing the disease (5-9). Environmental causes such as smoking and diets high in processed food, refined sugar, and milk protein have been theorized as explanations for this increase (10-14).
Additionally, patients with IBD have increased incidence of colon cancer and mortality from colon cancer when compared to the general population (15,16). Many population-based studies as well as meta-analyses around the world have corroborated this finding (17-19). Around the world, the incidence of colon cancer per year is 1.2 million and the mortality is 0.6 million per year (20).
Racial disparities in colon cancer in the general population are well established, with African-Americans having the highest incidence and mortality and Asians/Pacific Islanders having the lowest (21). Prior studies have analyzed gender and age of patients with IBD who develop colon cancer, demonstrating that overall, the risk of developing colon cancer is higher for men with IBD than for women with IBD and is higher for patients who are under the age of 40 (17,22). However, investigation of other demographic variables, such as race, in this subset of patients remains incomplete. This study seeks to determine whether there exists racial disparity in the incidence of colon cancer in patients with IBD. Findings from this study will help in identifying high risk patients who may develop CC and help shape public health efforts to ultimately reduce the incidence of colon cancer.
Methods
A retrospective analysis of the 2011 National Inpatient Sample (NIS) database was performed. This database is maintained by the Agency for Healthcare Research and Quality as part of the Healthcare Cost and Utilization Project (HCUP). It covers 95% of the US population and includes comprehensive abstracted discharged data. Inclusion criteria were age >18 years, diagnosis of IBD, and diagnosis of colon cancer. Patients were then stratified by race (Whites, Blacks, Hispanics, Asians/Pacific Islanders, and Native Americans), and gender. Age was also used as a variable. These categorical variables were then summarized by frequencies and the continuous variables were summarized by mean and standard deviation. Log-binomial regression was performed to derive relative risk (RR), while adjusting for age and gender, and then for IBD. The significance level was set at 5%. Our analysis was performed using SAS 9.4.
For this study, the use of NIS database conformed to the data-use agreement from HCUP. This study was reviewed by the University of Arizona, Institutional Review Board and was determined to be exempt from the need for approval.
Results
There was a total of 6,013,105 cases in the database that was analyzed in our study, of which 40.5% were male. Of these, 70.7% (n=4,250,073) of our total cases were classified as White, 15.7% (n=944,957) as Black, 10.9% (n=652,905) as Hispanic, 2.2% (n=130,472) as Asian/Pacific Islander, and 0.6% (n=34,698) as Native American (Table 1). Of all the patients sampled, 1.0% (n=57,542) had a diagnosis of IBD, 0.6% (n=33,501) had a diagnosis of colon cancer, and 0.005% (n=289) had a diagnosis of both. Out of all White patients, 1.1% (n=47,014) had a diagnosis of IBD, 0.6% (n=24,631) had a diagnosis of colon cancer, and 0.006% (n=256) had diagnosis of both. Zero-point-seven percent (n=6,668) of Black patients had a diagnosis of IBD, 0.6% (n=5,202) had a diagnosis of colon cancer, and 0.002% (n=17) had a diagnosis of both. For the Hispanic patients, 0.5% (n=3,155) had a diagnosis of IBD, 0.4% (n=2,668) had a diagnosis of colon cancer, and 0.002% (n=16) had a diagnosis of both. Out of all Asian and Pacific Islander patients, 0.4% (n=538) had a diagnosis of IBD, 0.7% (n=874) had a diagnosis of colon cancer, and 0% had a diagnosis of both. Lastly, out of all Native American patients, 0.5% (n=167) had a diagnosis of IBD, 0.4% (n=126) had a diagnosis of colon cancer, and 0% had a diagnosis of both. For all races, percent of cases that had a diagnosis of both IBD and colon cancer were calculated using the patients with IBD as the total.
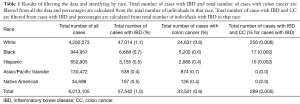
Full table
After adjusting for age, gender, and race in log-binomial regression, we found that out of all patients (both with and without IBD), increasing age (RR: 1.028, 95% CI: 1.027–1.028, P<0.0001), Black race (RR: 1.20, 95% CI: 1.17–1.24, P<0.0001), and Asian race (RR: 1.41, 95% CI: 1.32–1.51, P<0.0001) conferred an increased risk for developing colon cancer, whereas female gender (RR: 0.76, 95% CI: 0.75–0.78, P<0.0001), Hispanic race (RR: 0.958, 95% CI: 0.920–0.997, P=0.04), and Native American race (RR: 0.83, 95% CI: 0.69–0.98, P=0.03) conferred a decreased risk for developing colon cancer (Table 2). Interestingly, patients with IBD had a RR of 1.06 with a 95% CI of 0.94–1.19, that was not statically significant (P=0.34).
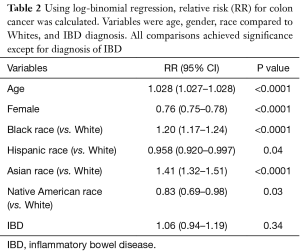
Full table
Out of patients with IBD only, increasing age (RR: 1.02, 95% CI: 1.01–1.03, P<0.0001) was the only statistically significant variable that conferred an increased risk of developing colon cancer, whereas female gender (RR: 0.56, 95% CI: 0.44–0.71, P<0.0001) and Black race (RR: 0.59, 95% CI: 0.36–0.98, P=0.04) conferred a decreased risk for developing colon cancer (Table 3). Hispanic race had a RR of 1.06 with a 95% CI of 0.64–1.76, that was not statically significant (P=0.81). Asian and Native American race were not applicable for this analysis since there were no cases with IBD and colon cancer in these populations.
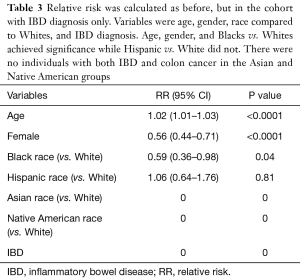
Full table
Discussion
Previous studies have shown that the overall incidence of colon cancer in patients with IBD is very low (23). This finding was confirmed in our study where out of all patients with IBD (57,542 patients), only 289 (0.005%) patients went on to develop colon cancer (Table 1). Interestingly, our study also showed that IBD was not a statistically significant risk factor for the development of colon cancer. Previous research has been mixed about whether IBD is a statistically significant predictor of risk of colon cancer development and thus it is not unusual that this variable was not significant in our study (24). Some studies have shown that IBD does statistically significantly increase the risk of colon cancer development, but others have found that it depends on which specific type and stage of IBD that is examined (24,25). Other studies corroborated our finding that IBD does not statistically significantly increase the risk of colon cancer and might even decrease the risk (26). This possible decrease in risk has been attributed to better screening for at-risk populations (26). These conflicting results from the various studies could be because UC and Crohn’s disease are lumped together and theses might have different impacts on carcinogenesis. It will be beneficial in future studies to distinguish between Crohn’s disease and UC as well as the stage of IBD to better tease out the relationship between IBD and the development of colon cancer.
We demonstrated in our study that advanced age, Black race, and Asian race were all statistically significant risk factors for colon cancer development, while Hispanic race, Native American race, and female gender all conferred a decreased risk of development of colon cancer (Table 2). Our findings on race and gender have been corroborated by previous studies (22,23).
We went on to analyze the incidence of colon cancer only in the patients with IBD. Our analysis showed that advanced age was still a statistically significant predictor of development of colon cancer. Female gender was still protective, which corroborates with findings from previous studies (17,22). Of the patients with IBD, Hispanics do not have a statistically significant increase or decrease in the development of colon cancer. However, being Black with IBD actually confers a statistically significant less chance of developing colon cancer. This is particularly interesting because Black race seems to have a protective effect on developing colon cancer for the patients with IBD, but an increased risk for the general population (both with IBD and without). While this finding is perplexing, one possible explanation is that Blacks with IBD may follow a more prudent surveillance given this population is known to be an at-risk group for colon cancer. However, this population has also been shown to have less access to healthcare. Another possible explanation for this disparity could be due to genetic predisposition. Blacks with IBD may possibly have a change in their gut flora that may be protective to development of colon cancer. Future studies are warranted to investigate change in the gut microbiome in patients of different races with IBD who develop colon cancer.
Our study has inherent limitations given that it is a retrospective analysis. Our data consisted of a large sample size, but the number of patients with IBD and colon cancer is small, which could impact the analysis. Another limitation is the fact that we could not separate the Crohn’s disease patients from the UC patients. This could not be done because the database did not separate them and grouped both under IBD.
Conclusions
Despite these limitations, our study showed that there are racial disparities in the incidence of colon cancer in patients with IBD. It also showed that a certain race could be both disadvantageous in one setting and protective in another. Future studies on larger and more specific databases will be useful to fully understand how race and ethnic groups impact the likelihood that patients with IBD will develop colon cancer. Additional studies on genetic predisposition will also help to better understand racial disparities. Shedding light on racial disparities will allow for focused healthcare efforts for at-risk populations to ultimately decrease the incidence and mortality of colon cancer.
Acknowledgements
We would like to thank the patients without whom this work would not be possible.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was reviewed by the University of Arizona, Institutional Review Board (IRB ID: 1608829801) and was determined to be exempt from the need for approval.
References
- Hanauer SB. Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflamm Bowel Dis 2006;12 Suppl 1:S3-9. [Crossref] [PubMed]
- Loftus EV Jr. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology 2004;126:1504-17. [Crossref] [PubMed]
- Whelan G. Epidemiology of inflammatory bowel disease. Med Clin North Am 1990;74:1-12. [Crossref] [PubMed]
- Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012;142:46-54.e42. [Crossref] [PubMed]
- Fielding JF. The relative risk of inflammatory bowel disease among parents and siblings of Crohn's disease patients. J Clin Gastroenterol 1986;8:655-7. [Crossref] [PubMed]
- Laharie D, Debeugny S, Peeters M, et al. Inflammatory bowel disease in spouses and their offspring. Gastroenterology 2001;120:816-9. [Crossref] [PubMed]
- Monsen U, Brostrom O, Nordenvall B, et al. Prevalence of inflammatory bowel disease among relatives of patients with ulcerative colitis. Scand J Gastroenterol 1987;22:214-8. [Crossref] [PubMed]
- Roth MP, Petersen GM, McElree C, et al. Familial empiric risk estimates of inflammatory bowel disease in Ashkenazi Jews. Gastroenterology 1989;96:1016-20. [Crossref] [PubMed]
- Mekhjian HS, Switz DM, Melnyk CS, et al. Clinical features and natural history of Crohn's disease. Gastroenterology 1979;77:898-906. [PubMed]
- Higuchi LM, Khalili H, Chan AT, et al. A prospective study of cigarette smoking and the risk of inflammatory bowel disease in women. Am J Gastroenterol 2012;107:1399-406. [Crossref] [PubMed]
- Persson PG, Ahlbom A, Hellers G. Diet and inflammatory bowel disease: a case-control study. Epidemiology 1992;3:47-52. [Crossref] [PubMed]
- Sakamoto N, Kono S, Wakai K, et al. Dietary risk factors for inflammatory bowel disease: a multicenter case-control study in Japan. Inflamm Bowel Dis 2005;11:154-63. [Crossref] [PubMed]
- Silverstein MD, Lashner BA, Hanauer SB, et al. Cigarette smoking in Crohn's disease. Am J Gastroenterol 1989;84:31-3. [PubMed]
- Tragnone A, Valpiani D, Miglio F, et al. Dietary habits as risk factors for inflammatory bowel disease. Eur J Gastroenterol Hepatol 1995;7:47-51. [PubMed]
- Bernstein CN, Nugent Z, Targownik LE, et al. Predictors and risks for death in a population-based study of persons with IBD in Manitoba. Gut 2015;64:1403-11. [Crossref] [PubMed]
- Ullman TA, Itzkowitz SH. Intestinal inflammation and cancer. Gastroenterology 2011;140:1807-16. [Crossref] [PubMed]
- Bernstein CN, Blanchard JF, Kliewer E, et al. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer 2001;91:854-62. [Crossref] [PubMed]
- Jess T, Rungoe C, Peyrin-Biroulet L. Risk of colorectal cancer in patients with ulcerative colitis: a meta-analysis of population-based cohort studies. Clin Gastroenterol Hepatol 2012;10:639-45. [Crossref] [PubMed]
- Kappelman MD, Farkas DK, Long MD, et al. Risk of cancer in patients with inflammatory bowel diseases: a nationwide population-based cohort study with 30 years of follow-up evaluation. Clin Gastroenterol Hepatol 2014;12:265-73.e1. [Crossref] [PubMed]
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: Globocan 2008. Int J Cancer 2010;127:2893-917. [Crossref] [PubMed]
- Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin 2014;64:104-17. [Crossref] [PubMed]
- Soderlund S, Granath F, Brostrom O, et al. Inflammatory bowel disease confers a lower risk of colorectal cancer to females than to males. Gastroenterology 2010;138:1697-703. [Crossref] [PubMed]
- Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin 2017;67:177-93. [Crossref] [PubMed]
- Jess T, Loftus EV Jr, Velayos FS, et al. Risk of intestinal cancer in inflammatory bowel disease: a population-based study from olmsted county, Minnesota. Gastroenterology 2006;130:1039-46. [Crossref] [PubMed]
- Herrinton LJ, Liu L, Levin TR, et al. Incidence and mortality of colorectal adenocarcinoma in persons with inflammatory bowel disease from 1998 to 2010. Gastroenterology 2012;143:382-9. [Crossref] [PubMed]
- Jess T, Simonsen J, Jorgensen KT, et al. Decreasing risk of colorectal cancer in patients with inflammatory bowel disease over 30 years. Gastroenterology 2012;143:375-81.e1; quiz e13-4. [Crossref] [PubMed]


