T4N0 colon cancers should be treated like T3N1 disease
Introduction
Patients with colon cancer that exhibit lymph node involvement have always been regarded to have more aggressive tumours with worse long-term outcome (1). In the 7th edition of the American Joint Committee on Cancer (AJCC) staging system for colorectal cancers, patients with lymph nodal involvement are classified to have stage III disease. Thus, these patients are strongly advised to undergo adjuvant chemotherapy and counselled accordingly.
On the other hand, chemotherapy is also offered to selected patients with stage II disease who are deemed to have high-risk features, such as the presence of lymphovascular or perineural involvement, poorly differentiated histology, emergency presentation with obstruction or perforation, <12 lymph nodes harvested, and T4 disease (2). Few papers have evaluated the relationship between T4N0 and T3N1 colon cancer. We therefore conducted this study to determine how does patients with T4N0 colon cancers compare to patients with T3N1 (stage III) disease by evaluating their long-term oncologic outcomes.
Methods
Data was obtained from a prospectively collected database in which preoperative, intraoperative and postoperative data was collected for all patients diagnosed with colorectal cancer in our unit. We included in this study patients who were diagnosed with colorectal cancer from January 2008 to December 2014. Ethics approval by the National Healthcare Group (NHG) Domain Specific Review Board (DSRB) was obtained. Informed consent from individual patients was not obtained for this study as this was a retrospective analysis without any further intervention. Most patients had also been discharged from follow-up, precluding the obtaining of consent. No funding was required for this study.
All patients were staged according to the 6th or 7th editions of the AJCC manual for colon cancer, depending on the most recent edition present at time of diagnosis. Patients were excluded if they had metastatic disease (M1), had a history of familial colorectal cancer syndromes or if the cancer was at or distal to the rectosigmoid junction. All consecutive patients with T3N1 and T4N0 disease were included in this study.
Preoperative and demographic information were analysed. This included the patient’s age, gender, race, site of tumour (left or right), pre-resection carcinoembryonic antigen (CEA) level, American Society of Anaesthesia (ASA) level, as well as if the patient presented with obstruction or perforation. Intraoperative variables analysed included the nature of surgery (elective or emergency), whether a stoma was created and type of operation (open, laparoscopic, robotic or converted).
Thirty-day complications following surgery were also collected, including the presence of surgical complications such as surgical site infections, anastomotic leak, and other postoperative complications. Histological details were analysed for grade of cancer, involvement of radial and longitudinal margins, vascular or lymphatic invasions and total lymph nodes collected. Patients were also analysed for whether they received adjuvant chemotherapy.
Patients were followed up at the outpatient surgical clinic and in concordance to guidelines by American Gastroenterology Association (3). All patients received regular history and physical examination, endoscopic as well as radiologic investigations to look for disease recurrences (3).
Caecal, ascending colon, and transverse colon tumours were defined as right sided cancers, and descending colon and sigmoid colon tumours were defined as arising from the left.
Univariable comparisons of categorical data between groups were performed by Chi-square test, while continuous data were analysed by Student’s t-test. Following univariate analysis, variables which were found to have P value <0.05 were selected for evaluation via multivariate analysis by logistic and linear regression. Overall survival outcomes were analysed by the Kaplan-Meier method, whilst variables were analysed for earlier time to death by Cox regression analysis. A P value <0.05 was considered to be statistically significant. All analyses were performed by Stata software, version 14 (StataCorp, College Station, TX, USA).
Results
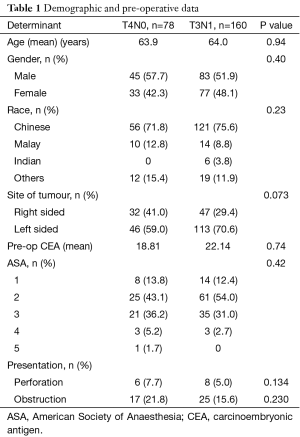
Between 2008 and 2014, there were 78 (32.8%) patients with T4N0 disease, and 160 (67.2%) patients with T3N1 disease (Table 1) that were managed at our institution. Demographic and preoperative determinants such as age, gender, site of tumour, CEA levels, ASA score and presentation with obstruction or perforation were not statistically significant between patients with T3N1 and T4N0 disease. Patients with T4N0 disease were followed up for a median of 41.4 (range, 21.6–65.0) months, whilst patients with T3N1 disease were followed up for a median of 42.4 (range, 21.1–63.8) months.
Full table
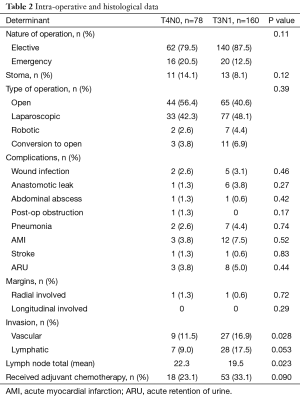
Intraoperative variables were not found to be statistically significant between both groups (Table 2). The nature and type of surgery was comparable between both groups, as were the creation of a stoma. Both surgery specific as well as systemic complications were also not statistically significant. At histological analysis, when comparing T4N0 and T3N1 cases, there were statistically significant differences in the vascular involvement (11.5% vs. 16.9% respectively, P=0.028), and mean total lymph nodes harvested (22.3 vs. 19.5 respectively, P=0.023). The difference in the presence of lymphatic invasion between both groups was found to approach statistical difference with a P value of 0.053. As the presence of lymphatic invasion could potentially lead to worse outcomes for the patient, it was included in multivariate analysis even though it did not meet the <0.05 cut-off. The proportion of patients in each group undergoing chemotherapy was also similar with no statistical difference identified. As with the presence of lymphatic invasion, we felt that this would also affect outcomes and hence was included in multivariate analysis in spite of P value being 0.090.
Full table
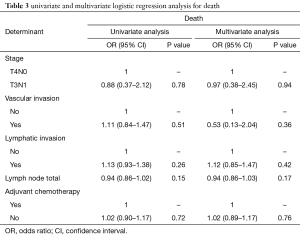
Vascular invasion and total lymph node yield were also identified as variables for further analysis on the basis of achieving significance of P<0.05. Together with lymphatic invasion, adjuvant chemotherapy, and stage of tumour (T4N0 vs. T3N1), these were therefore included in univariate analysis for survival (Table 3).
Full table
When analysed for death as an outcome, none of the variables (T3N1 vs. T4N0, vascular invasion, lymphatic invasion, adjuvant chemotherapy) achieved statistical significance on univariate analysis. Multivariate analysis was repeated with these variables and none of the variables achieved statistical significance for death as well.
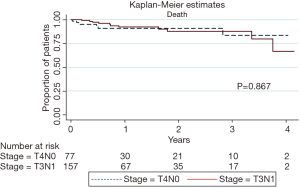
Kaplan-Meier analysis was then performed and highlighted similar survival rates (P=0.867) (Figure 1) amongst patients with T4N0 and T3N1 disease. Cox regression analysis was used to evaluate the prognostic variables affecting survival (Table 4). Univariate and multivariate analysis showed that stage, vascular invasion, lymphatic invasion, number of lymph nodes harvested and receiving adjuvant chemotherapy were all not statistically significant factors affecting survival.

Full table
Discussion
Our results of 238 patients highlighted that patients with T4N0 tumours fare very similarly to patients with T3N1 tumours. There was no difference in the association between stage of disease (T4N0 or T3N1) and overall survival (OS: 0.97 in T3N1, P=0.94), even when accounting for other factors such as vascular or lymphatic invasion, lymph node yield, or administration of adjuvant chemotherapy. Kaplan-Meier survival curves demonstrated no difference between T4N0 or T3N1 cancer. The association between time to death as measured by hazard ratios were also not significant (OS: 0.56 in T3N1, P=0.26). This led us to advocate that patients with stage II T4N0 disease should be advised and treated as for patients with T3N1 disease.
Some authors have postulated that the poorer survival in patients with T4N0 disease is due to inadequate lymph node yield or the administration of chemotherapy in patients with stage III disease (4,5). In our study, mean lymph node yield was actually higher in the group of patients with T4N0 disease (22.3 vs. 19.5, P=0.023), suggesting that under staging of disease due to insufficient or inadequate lymph node yield is not the reason for a poorer prognosis in T4N0 disease. Furthermore, on both univariate and multivariate analysis of lymph node yield, there was no evidence for the association between yield and overall survival. Our findings are supported by evidence which suggests that yield alone cannot account for improved cancer survival after adequate lymph node yield have been obtained (6). The presence of under staging or inadequate lymphatic clearance in stage II colon cancers have been shown to be a significant factor only if fewer than 12 lymph nodes have been removed for analysis in colon cancers (7).
The administration of chemotherapy may however have a role to play in the poorer prognosis amongst T4N0 patients. In our study, we noted a non-significant increased proportion of patients in the T3N1 group who had undergone chemotherapy compared with the T4N0 group (33.1% vs. 23.1%, P=0.090). The authors believed that this is due to the traditional thinking that T4N0 disease is a stage II disease and hence advocating chemotherapy in these patients may not be as aggressive or easily accepted by oncologists and patients alike. The authors also acknowledge the low chemotherapy uptake rate for stage III patients of 33.1% in our study compared with chemotherapy uptake rates of 55–80% in the literature (8,9). We postulate that this is due to misinformation about chemotherapy and fears regarding the chemotherapy regime in our local population resulting in the low uptake rate. Our study group is currently conducting a study to address this concern, with the aim of debunking any misperceptions about chemotherapy in our local population.
Interestingly, after controlling for stage of disease, vascular and lymphatic invasion, as well as lymph node yield, the odds ratio for death in patients who did not receive adjuvant chemotherapy was no different than patients who did [odds ratio (OR) 1.02, P=0.76]. Rather than suggesting that there is no use for chemotherapy, we believe this suggests that better identification of patients who would benefit from chemotherapy needs to be achieved so as to reap the maximum benefit for it.
On a separate note, some have suggested that T4 tumours may intrinsically exhibit a different tumour biology which leads to worse outcomes, necessitating a shift in the management paradigm of patients with T4 disease (10). Molecular and genetic markers have been proposed targeting patients with stage II disease, allowing for more personalised medicine (11-13). CDX2 is one such genetic marker which has been found to be associated with more aggressive colon cancer in patients with stage II disease, but also predicts for better response to chemotherapeutic agents (14).
Finally, we also opted not to compare T4N0 with T3N0 colon cancers as there is conclusive evidence in the literature on the worse long-term oncological outcomes in terms of overall survival (70% vs. 74%, P=0.02) and overall recurrence rates (26.5% vs. 16.4%, P=0.02) (10).
There were several limitations to our study which included its retrospective nature and the possibility of selection bias. However, our study remained important to highlight the similarity in the outcomes amongst patients with T4N0 and T3N1 disease. More work remains to be done to seriously elucidate if it is the tumour biology, extent of mutation, response to chemotherapeutic agents and many other yet unknown factors that could account for the less than ideal outcomes in these patients.
Conclusions
T4N0 colon cancers have similar outcomes to T3N1 disease and should be considered as stage III disease in future classification. Patients diagnosed with T4N0 disease should receive similar treatment as those with T3N1 disease and counselled accordingly on the prognosis.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Ethics approval by the National Healthcare Group (NHG) Domain Specific Review Board (DSRB, No. 2015/00842) was obtained. Informed consent from individual patients was not obtained for this study as this was a retrospective analysis without any further intervention.
References
- Muralidhar V, Nipp RD, Ryan DP, et al. Association Between Very Small Tumor Size and Increased Cancer-Specific Mortality in Node-Positive Colon Cancer. Dis Colon Rectum 2016;59:187-93. [Crossref] [PubMed]
- Varghese A. Chemotherapy for Stage II Colon Cancer. Clin Colon Rectal Surg 2015;28:256-61. [Crossref] [PubMed]
- American Gastroenterology Association. AGA institute guidelines for colonoscopy surveillance after cancer resection: clinical decision tool. Gastroenterology 2014;146:1413-4. [Crossref] [PubMed]
- O'Connell JB, Maggard MA, Ko CY. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst 2004;96:1420-5.
- Jeong SY, Chessin DB, Schrag D, et al. Re: Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst 2005;97:1705-6; author reply 1706-7.
- van Erning FN, Crolla RM, Rutten HJ, et al. No change in lymph node positivity rate despite increased lymph node yield and improved survival in colon cancer. Eur J Cancer 2014;50:3221-9. [Crossref] [PubMed]
- Stracci F, Bianconi F, Leite S, et al. Linking surgical specimen length and examined lymph nodes in colorectal cancer patients. Eur J Surg Oncol 2016;42:260-5. [Crossref] [PubMed]
- Dobie SA, Baldwin LM, Dominitz JA, et al. Completion of therapy by Medicare patients with stage III colon cancer. J Natl Cancer Inst 2006;98:610-9. [Crossref] [PubMed]
- Jessup JM, Stewart A, Greene FL, et al. Adjuvant chemotherapy for stage III colon cancer: implications of race/ethnicity, age, and differentiation. JAMA 2005;294:2703-11. [Crossref] [PubMed]
- Rottoli M, Stocchi L, Dietz DW. T4N0 colon cancer has oncologic outcomes comparable to stage III in a specialized center. Ann Surg Oncol 2012;19:2500-5. [Crossref] [PubMed]
- Uen YH, Lin SR, Wu DC, et al. Prognostic significance of multiple molecular markers for patients with stage II colorectal cancer undergoing curative resection. Ann Surg 2007;246:1040-6. [Crossref] [PubMed]
- Meyer A, Merkel S, Brückl W, et al. Cdc2 as prognostic marker in stage UICC II colon carcinomas. Eur J Cancer 2009;45:1466-73. [Crossref] [PubMed]
- Poincloux L, Durando X, Seitz JF, et al. Loss of Bcl-2 expression in colon cancer: a prognostic factor for recurrence in stage II colon cancer. Surg Oncol 2009;18:357-65. [Crossref] [PubMed]
- Dalerba P, Sahoo D, Paik S, et al. CDX2 as a Prognostic Biomarker in Stage II and Stage III Colon Cancer. N Engl J Med 2016;374:211-22. [Crossref] [PubMed]