Multimodality management of incidentally detected gall bladder cancer: long term results from a tertiary care cancer centre
Introduction
Gall bladder cancer (GBC) is one of the common malignancies of the gastrointestinal tract and is the most common biliary tract malignancy in India having an age standardised incidence rate of 1.8 per 100,000 population (1). The patients with GBC present either with incidental diagnosis after simple cholecystectomy (SC) or with a primary gall bladder mass. Incidental cancer of gall bladder (ICGB) is defined as carcinoma of gall bladder diagnosed during or after SC for benign gallbladder disease. ICGB is reported in 0.2–2.9% of patients undergoing SC (2,3). It has traditionally been thought to be a relatively early stage disease but there are controversies associated with various aspects of revision surgery. Role of adjuvant therapy in ICGB is not clearly defined and multi-modality management is still evolving. In this article we describe our experience and results with multimodality management of ICGB.
Methods
A retrospective analysis of incidentally detected GBC patients operated between 2002 and 2012 was performed from the prospectively maintained clinical database in the Department of Surgical Oncology.
Treatment protocol
All patients presenting with ICGB were evaluated in a multidisciplinary clinic. The records of ultrasound done before SC, operative notes and pathology report were reviewed at presentation. A contrast enhanced computerized tomography (CECT) abdomen was done to assess the residual disease and operability. Diagnostic laparoscopy or diagnostic mini-laparotomy (through subcostal approach) was planned to assess metastatic disease. A revision surgery was performed in all resectable patients, which included wedge resection of segment IVB and V (with at least 2 cm margin of liver parenchyma), lymph node dissection of the hepato-duodenal ligament, retro-duodenal/retro-pancreatic and coeliac nodes along with excision of port sites. Inter aorto-caval nodes were dissected first and were checked for metastasis through frozen section. The procedure was abandoned if frozen section of inter aorto-caval nodes were positive for metastasis. American Joint Committee on Cancer (AJCC) Cancer Staging Manual, 7th edition, 2010 was used for final staging (4). All patients with tumor stage ≥ T1b or nodal involvement were recommended for concurrent chemoradiation with 5-Fluoro uracil and Folinic acid (total of 6 cycles) along with 45 Gy/25 fractions of external beam radiotherapy. Patients were followed-up at 3 monthly intervals with clinical examination and CECT abdomen if there were any suspicion of recurrence.
Data compilation and statistical analysis
The data were prospectively entered in predesigned Microsoft access database and updated during regular audits. The last follow-up information was completed either through the medical records or by telephonic enquiry. All the statistical analysis was done using STATA 14 software (StataCorp. 2016. Stata Statistical Software: Release 14.2 College Station, TX: StataCorp LP). The survival analysis was performed using Kaplan-Meir method and Cox regression was used for prognostic factor analysis. Univariate and multivariate analysis were done to determine the prognostic variables.
Results
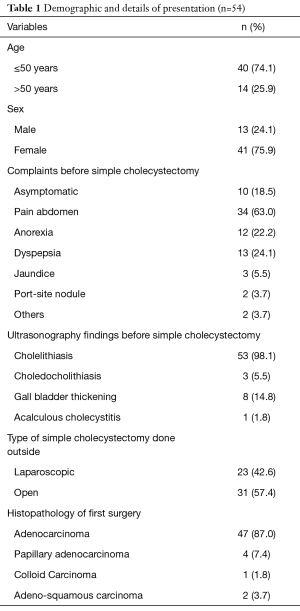
Study population comprised of 54 patients diagnosed with incidentally detected GBC after SC done outside. The male to female ratio was 1:3. The mean age at presentation was 47.5 (32.0–63.0) years. A total of 31 (57.4%) and 23 (42.6%) patients, respectively had undergone open and laparoscopic SC elsewhere before presentation. The clinical details, imaging findings and operative findings at previous SC are depicted in Table 1. The median time interval between SC and revision surgery was 65 days.
Full table
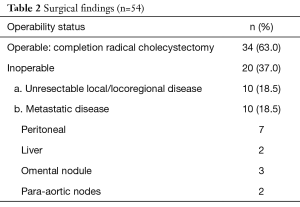
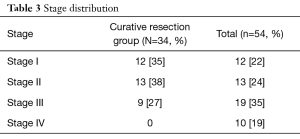
The details of surgical findings are depicted in Table 2. The mean blood loss was 150 ml and mean operative time was 205 minutes. Eleven patients had dense fibrosis in the GB fossa, out of which one patient was inoperable in view of frozen porta. There was no peri-operative mortality. Post-operative complications included surgical site infection in 5 patients (9.26%) followed by prolonged ileus in 3 (5.5%), bile leak in 2 (3.7%) and delayed stricture of bile duct in one patient. Mean number of lymph nodes harvested was 7.4 (2.0–18.0). In the curatively resected group, residual disease in GB fossa and lymph node were present in 7 (20%) patients and 4 (11.4%) patients respectively. One patient had port site metastasis. Resection margin was positive in one patient. Final TNM stage details are depicted in Table 3. A total of 18 patients received adjuvant chemo-radiotherapy and 12 could complete the planned regimen.
Full table
Full table
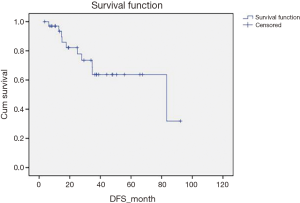
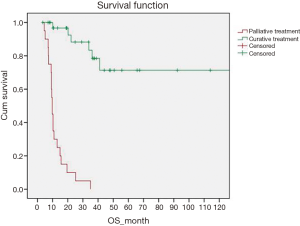
After a median follow-up of 36.5 months a total of 10 (18.5%) patients had recurrence; 2 local, 3 loco-regional and 5 systemic. The 5-year overall survival (OS) of all patients was 42%. The 5 years disease free survival (DFS) and OS for patients receiving curative treatment was 64% and 72% respectively (Figures 1,2). Stage wise distribution of OS for patients curatively treated was 97% (stage I), 62% (stage II) and 52% (stage III). On univariate analysis, presence of residual disease in the gallbladder fossa was a significant risk factor for disease recurrence (hazard ratio 11.9, P=0.007). Adjuvant chemoradiation received (P=0.003) and stage (P=0.016) were significant prognostic factors for OS.
Discussion
For a long time GBC has been labelled as a very lethal disease with vast majority of patients presenting at advanced stage with dismal prognosis leading to a state of nihilism prevalent for decades amongst the medical fraternity. The incidence of ICGB is increasing in last few decades due to increasing number of cholecystectomies done (5).
Ultrasound findings of a polyp, focal irregular gall bladder wall thickness, any non-dependent solid lesion should raise a suspicion of underlying carcinoma and should be planned accordingly as per surgery for GBC. In community surgical settings, some patients are explored for SC even after a suspicion for GBC is there on ultrasound (6). In the present study, review of the previous ultrasound film and/or report showed 8 (14.8%) patients had suspicion of GBC.
Laparoscopic cholecystectomy is gold standard for benign gall bladder disease and thus in recent years, ICGB are seen more commonly after laparoscopy than after open cholecystectomy. The concerns of poor outcome in ICGB due to increased manipulation and spillage in laparoscopic than open cholecystectomy is refuted by recent data (7). However, in developing countries open surgery has continued to be performed in community hospitals till late and in the present study 57.4% patients had undergone previous open cholecystectomy.
The importance of a good operative note and pathology report in the SC specimen cannot be overemphasized, because the specimen is usually not available before revision surgery. The operative note should include the details of indication for surgery, presence or suspicion of mass, location of mass (fundus, body, neck or cystic duct), any stones, size, condition of serosa, status of surrounding organs, any difficulty in dissection of gall bladder bed, extent of adhesions, cystic duct status, enlarged cystic and periportal nodes, any unwanted bleeding, bile spillage, perforation of gall bladder, method of retrieval of specimen, port used for retrieval, any conversion and reason for conversion. The surgeon should open all specimens after SC, to see if there is any thickening of wall, polyp or mass in gall bladder. If there is a gall bladder mass or any finding with suspicion of malignancy, frozen biopsy of the specimen should be done. If frozen biopsy is positive for malignancy, revision surgery should be performed in the same sitting if expertise is available. All SC specimens must be subjected to histopathological examination to avoid any subjective variation although some groups have questioned the utility of routine histopathology of specimens advocating focussed examination of suspicious specimens only (8-11).
While there are no randomised trials to establish the role and extent of re-resection in ICGB, there are many retrospective and prospective cohort studies, describing the outcomes of re-resection in different T stages. The optimal management of T1a disease is straightforward with SC that has already been done in ICGB. There is no proven benefit of re-resection in T1a disease in terms of recurrence rate and survival (12). The rate of lymph nodal metastasis in T1a disease is 2.5% and extended cholecystectomy is not indicated for these patients (13).
The surgical approach, whether to perform SC or revision surgery in T1b disease is more controversial. A high rate of residual disease is found in lymph nodes (up to 20%) and liver bed disease (up to 13%) in T1b disease (14-16). The data from German cancer registry suggested higher recurrence with SC; of which 50% occurred in the lymph nodal basin. In another study the 5-year survival was 79% and 42% for patients with or without re-resection (P=0.03). Higher recurrence (24.5% vs. 8.6%) was found in patients who didn’t undergo re-resection (12). The authors concluded that, revision surgery including regional lymphadenectomy is justified and preferred because of the possibility of occult lymph node metastasis and for accurate assessment of stage. In our study the incidence of lymph nodal involvement in T1b tumors was 14%.
Revision cholecystectomy is recommended in all T2 and T3 tumors. The aim of resection of the liver bed in ICGB is to re-excise the gall bladder bed to ensure R0 resection. There is no consensus regarding the extent of resection of the gall bladder bed. Different options available in resectable patients are wedge resection of segment IVB and V, segmentectomy of IVB and V and right hemi-hepatectomy. Survival results of these procedures are similar for T1b and T2 disease. We followed the policy of wedge resection with at least 2 cm margin of liver parenchyma. Major hepatectomies are done for tumours with extensive liver infiltration but prognosis of this group of patients is poor (14-17). Neo-adjuvant chemotherapy followed by surgery may be an option for advanced disease (18). Common bile duct resection may be required for tumours located in the neck region or in cases where cystic duct margin was involved by tumour. Routine resection of extra-hepatic bile duct (EHBD) is not recommended, except to achieve an R0 resection or in case of devascularisation of a segment of CBD as a result of extensive nodal dissection (19). We revised the cystic duct stump and it was sent separately for histopathology evaluation.
The operability rate of ICGB, after they are found resectable on imaging, is variable (12,18,20-22). The present series had an operability rate of 65%. Most common cause of inoperability was presence of peritoneal and liver metastasis. Apart from these, fibrosis at porta was another important factor leading to inoperability. We grouped the fibrosis at the porta, into mild, moderate and severe fibrosis, according to the operating surgeon’s assessment. This may be related to the timing of the revision surgery. With increasing time after SC, there is increased fibrosis at the porta. Other factors which may be associated with increased fibrosis are history of cholecystitis, bile spillage during SC, injury to surrounding organs. Revision surgery should be done as early as possible. A study from India reported an overall resectability rate of 58.4%. Staging laparoscopy identified 94.1% of the inoperable lesions and rest detected after laparotomy (23).
Residual disease after revision surgery has been reported in up to 32% in gall bladder fossa and 46% in lymph nodes (20,23). In the current study presence of residual disease either in the gall bladder fossa or lymph nodes was found to be associated with poor prognosis. None of the patients with residual disease in gall bladder fossa survived beyond 3 years. Port site may be a potential site for residual disease and we routinely perform the excision of port-site or previous scar along-with revision surgery. Most common site of recurrence after revision surgery in GBC is usually distant metastasis. In the present study liver was the most common site of metastasis followed by para aortic lymph nodes. Other studies have also shown similar results (24-28).
Although the role of adjuvant chemoradiation in GBC is debatable as most of the current literature describes the multimodality management in context of tumors of hepato-biliary origin where the proportion of GBC remains variable (29,30). In the present study it was found to be a significant prognostic factor associated with better OS. A number of issues including modality and regimen need to be examined through randomized studies.
Conclusions
Incidentally detected GBC is increasing in incidence. A multi-modality approach with revision surgery and adjuvant chemoradiation treatment may yield better outcome. Presence of residual disease is a poor prognostic factor. Optimal evaluation before SC and early referral to specialty center is therefore important in patients with suspicion of gallbladder malignancy because first chance is probably the best chance.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ferlay J. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer, 2013. Available online: http://globocan.iarc.fr
- Toyonaga T, Chijiiwa K, Nakano K, et al. Completion radical surgery after cholecystectomy for accidentally undiagnosed gallbladder carcinoma. World J Surg 2003;27:266-71. [Crossref] [PubMed]
- Yamamoto H, Hayakawa N, Kitagawa Y, et al. Unsuspected gallbladder carcinoma after laparoscopic cholecystectomy. J Hepatobiliary Pancreat Surg 2005;12:391-8. [Crossref] [PubMed]
- Edge SB, Byrd DR, Compton CC, et al. American Joint Committee on Cancer Staging Manual. 7th edition. New York: Springer, 2010.
- Behari A, Kapoor VK. Asymptomatic Gallstones (AsGS) – To Treat or Not to? Indian J Surg 2012;74:4-12. [Crossref] [PubMed]
- Pitt SC, Jin LX, Hall BL, et al. Incidental gallbladder cancer at cholecystectomy: when should the surgeon be suspicious? Ann Surg 2014;260:128-33. [Crossref] [PubMed]
- Goetze TO, Paolucci V. Prognosis of incidental gallbladder carcinoma is not influenced by the primary access technique: analysis of 837 incidental gallbladder carcinomas in the German Registry. Surg Endosc 2013;27:2821-8. [Crossref] [PubMed]
- Jayasundara JA, de Silva WM. Histological assessment of cholecystectomy specimens performed for symptomatic cholelithiasis: routine or selective? Ann R Coll Surg Engl 2013;95:317-22. [Crossref] [PubMed]
- van Vliet JL, van Gulik TM, Verbeek PC. Is it necessary to send gallbladder specimens for routine histopathological examination after cholecystectomy? The use of macroscopic examination. Dig Surg 2013;30:472-5. [Crossref] [PubMed]
- Deng YL, Xiong XZ, Zhou Y, et al. Selective histology of cholecystectomy specimens-is it justified? J Surg Res 2015;193:196-201. [Crossref] [PubMed]
- Mittal R, Jesudason MR, Nayak S. Selective histopathology in cholecystectomy for gallstone disease. Indian J Gastroenterol 2010;29:32-6. [Crossref]
- Goetze TO, Paolucci V. Immediate re-resection of T1 incidental gallbladder carcinomas: a survival analysis of the German Registry. Surg Endosc 2008;22:2462-5. [Crossref] [PubMed]
- Ogura Y, Mizumoto R, Isaji S, et al. Radical operations for carcinoma of the gallbladder: present status in Japan. World J Surg 1991;15:337-43. [Crossref] [PubMed]
- You DD, Lee HG, Paik KY, et al. What is an adequate extent of resection for T1 gallbladder cancers? Ann Surg 2008;247:835-8. [Crossref] [PubMed]
- Fong Y, Jarnagin W, Blumgart LH. Gallbladder Cancer: Comparison of Patients Presenting Initially for Definitive Operation With Those Presenting After Prior Noncurative Intervention. Ann Surg 2000;232:557-69. [Crossref] [PubMed]
- Pawlik TM, Choti MA. Biology Dictates Prognosis Following Resection of Gallbladder Carcinoma: Sometimes Less is More. Ann Surg Oncol 2009;16:787-8. [Crossref] [PubMed]
- D’Angelica M, Dalal KM, DeMatteo RP, et al. Analysis of the extent of resection for adenocarcinoma of the gallbladder. Ann Surg Oncol 2009;16:806-16. [Crossref] [PubMed]
- Sirohi B, Mitra A, Jagannath P, et al. Neoadjuvant chemotherapy in patients with locally advanced gallbladder cancer. Future Oncol 2015;11:1501-9. [Crossref] [PubMed]
- Shukla PJ, Barreto S. Systematic review: Should routine resection of the extra-hepatic bile duct be performed in gallbladder cancer? Saudi J Gastroenterol 2010;16:161. [Crossref] [PubMed]
- Bartlett DL, Fong Y, Fortner JG, et al. Long-term results after resection for gallbladder cancer.Implications for staging and management. Ann Surg 1996;224:639-46. [Crossref] [PubMed]
- de Aretxabala X, Roa I, Burgos L, et al. Gallbladder cancer in Chile. A report on 54 potentially resectable tumors. Cancer 1992;69:60-5. [Crossref] [PubMed]
- Muratore A, Polastri R, Bouzari H, et al. Radical surgery for gallbladder cancer: a worthwhile operation? Eur J Surg Oncol 2000;26:160-3. [Crossref] [PubMed]
- Agarwal AK, Kalayarasan R, Javed A, et al. The Role of Staging Laparoscopy in Primary Gall Bladder Cancer-An Analysis of 409 Patients: A Prospective Study to Evaluate the Role of Staging Laparoscopy in the Management of Gallbladder Cancer. Ann Surg 2013;258:318-23. [Crossref] [PubMed]
- Wiggers JK, Groot Koerkamp B, Ovadia Z, et al. Patterns of recurrence after resection of gallbladder cancer without routine extrahepatic bile duct resection. HPB 2014;16:635-40. [Crossref] [PubMed]
- Park JS, Yoon DS, Kim KS, et al. Actual recurrence patterns and risk factors influencing recurrence after curative resection with stage II gallbladder carcinoma. J Gastrointest Surg 2007;11:631-7. [Crossref] [PubMed]
- Kim WS, Choi DW, You DD, et al. Risk factors influencing recurrence, patterns of recurrence, and the efficacy of adjuvant therapy after radical resection for gallbladder carcinoma. J Gastrointest Surg 2010;14:679-87. [Crossref] [PubMed]
- Jung SJ, Woo SM, Park HK, et al. Patterns of initial disease recurrence after resection of biliary tract cancer. Oncology 2012;83:83-90. [Crossref] [PubMed]
- Jarnagin WR, Ruo L, Little SA, et al. Patterns of initial disease recurrence after resection of gallbladder carcinoma and hilar cholangiocarcinoma. Cancer 2003;98:1689-700. [Crossref] [PubMed]
- Gold DG, Miller RC, Haddock MG, et al. Adjuvant therapy for gallbladder carcinoma: the Mayo Clinic Experience. Int J Radiat Oncol Biol Phys 2009;75:150-5. [Crossref] [PubMed]
- Horgan AM, Amir E, Walter T, et al. Adjuvant therapy in the treatment of biliary tract cancer: a systematic review and meta-analysis. J Clin Oncol 2012;30:1934-40. [Crossref] [PubMed]