
A phase I trial of Proton stereotactic body radiation therapy for liver metastases
Introduction
Stereotactic body radiation therapy (SBRT) delivers an ablative regimen of highly focused external beam radiotherapy to target one or more discrete extracranial lesions. Multiple retrospective and prospective studies using SBRT for the treatment of metastatic liver disease have been reported in the literature (1). Published reports using SBRT to treat liver metastases have shown actuarial local control rates ranging from 50–100% (1,2) with higher doses associated with better local control (3). A multi-institutional phase I/II study of SBRT for liver metastases showed the safety of dose escalation from 36 up to 60 Gy in 3 fractions with a 2-year actuarial in-field local control rates of 92% (4). Lesions smaller than 3 cm had a 100% local control at 2 years. No randomized phase III data have been reported, but RAS01 (5), an international multicenter randomized trial comparing radiofrequency ablation with SBRT in lesion smaller than 4 cm, is open and actively recruiting.
In patients with metastatic disease to the liver, aggressive local therapy using modern radiotherapy techniques are promising and project to have a substantial role. Overall SBRT appears to be a safe and effective option for local control in the liver. From this work, we have also learned that these patients often develop a recurrence elsewhere in the liver. Joo et al. noted a 59% “out of field” recurrence rate (6). Because it is so common for out of field recurrences, the need for subsequent therapy often becomes necessary.
The use of Proton vs. X-ray for SBRT is attractive given the advantage of a lack of an exit dose (7,8) (Figure 1). The exit dose is responsible for high integral dose of radiation in the liver. This significantly limits the ability to administer subsequent courses. Only one Proton SBRT liver metastases paper has been published to date (9). Though toxicity was found to be minimal, the local control was slightly lower than other contemporary series. The author notes this is likely to be due to a low biologically equivalent dose (BED) used, where the maximum dose was 10×5 fx. They surmise that dose escalation may be a potential strategy to improve local control.
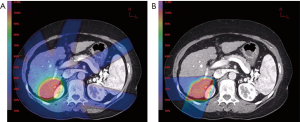
This is the first phase I dose escalation publication evaluating Proton SBRT. This phase I study was conducted to determine the feasibility and maximum tolerated dose (MTD) of Proton SBRT in patients with liver metastases in anticipation of a subsequent phase II trial to determine efficacy. The dosimetric advantage of proton therapy may lead to improved clinical outcomes with less morbidity. Importantly, as out of field recurrences are so common, Proton SBRT also affords opportunity for subsequent courses.
Methods
Eligibility
The study protocol was approved by our Institutional Review Board and informed consent was obtained for all patients. Patients treated included non-surgical candidates, or those who declined surgery for liver metastases. Inclusion criteria included patients with 1–3 liver metastases, <5 cm individual diameter irrespective of combined diameter, ECOG ≥1, life expectancy ≥6 months. Patients were required to have adequate liver reserve defined as total bilirubin <5 mg/dL, albumin >2.0 g/dL, liver enzymes <5 times the upper limit of normal, and an international normalized ratio (INR) less than 1.5. Kidney reserve was also required and defined as serum creatinine <2 mg/dL. Ascites requiring paracentesis was not permitted. Chemotherapy and/or targeted therapy were held at least 2 weeks before and 2 weeks after completion of SBRT. Any modality of prior liver therapy was allowed, assuming the above was met and that there were no overlapping radiation fields.
Primary objective
To identify the MTD of Proton SBRT in patients with metastatic liver disease in anticipation of subsequent phase II study.
Dose escalation
Dose escalation was conducted in a standard phase I design in an effort to identify the MTD. Three dose cohorts were preselected: a low dose cohort 12×3 fractions, intermediate dose cohort 16×3 fractions and high dose cohort 20 Gy ×3 fractions.
Toxicity was graded according to Common Terminology Criteria for Adverse Events (CTCAE) 4.0. Dose-limiting toxicities (DLT) included radiation induced liver disease (RILD). RILD is a clinical syndrome of anicteric ascites, hepatomegaly and elevation of alkaline phosphatase (ALP) relative to other transaminases that may occur 2 weeks to 3 months following radiation to the liver. Grade 3 ALP (>5 times upper limit of normal) with ascites not due to cancer progression was considered RILD.
Other DLTs included any grade 4 adverse event related to treatment protocol of any organ system within 90 days from the start of treatment. Each dose group was planned to consist of 3 patients unless 1 had a DLT. In that case, a second cohort of 3 patients would be added and await 90 days before moving to the next dose group. The MTD was defined as the dose level below which results in 2 or more of the 6 patients having a DLT.
Proton SBRT technique and dose specifications
Patients were immobilized during computed tomography (CT) simulation with full body pod immobilization, as previously described by Wroe et al. (10), with respiratory control via a volume breath hold device (SDX, Qfix, Avondale, PA, USA). Gross tumor volume (GTV) was defined to encompass the enhancing/hypoattenuating lesion on the contrast portion of the CT scan. Clinical target volume (CTV) was considered the same as the GTV. The CTV was then expanded by a 5 mm craniocaudal margin to create the internal target volume (ITV) to account for variability in breath hold position. A two to three beam, computer-generated treatment plan was created to deliver the prescription dose in 3 equal fractions over 3–5 days. During the planning process beam energy, modulation, aperture and bolus size/shape were optimized to account for beam uncertainty and daily positioning variation. Whenever possible beam orientations were chosen to avoid ending on bowel.
Normal tissue dose constraints
The liver constraint was that at least 700 cc of normal liver must receive <15 Gy (V<15 at least 700 cc) (11). Additionally, no more than 30% of the normal liver can receive more than 27 (V27 <30%) and no more than 50% of normal liver may receive more than 24 (V24 <50%). At least 67% of the right kidney must receive a total dose of <15 (V<15 at least 67%). Heart/pericardium maximum permitted dose was 30. Chest wall, stomach, esophagus or small intestine was limited to maximum dose of 30 at any point. The maximal dose to any point within the spinal cord could not exceed 18. Image-guided therapy using 2D orthogonal X-rays aligned to boney anatomy, and breath hold using SDX during treatment delivery was used for all patients.
Follow-up
Patients were planned to be evaluated once during the treatment course, at 4 weeks after treatment and at 3 months after treatment. Evaluation included physical exam, labs, follow-up imaging (starting at 3 months), and a detailed account of possible symptoms. Any observed toxicity within 90 days of treatment were recorded as acute toxicity. Regular follow-up was planned to continue at 3-month intervals to evaluate recurrence or progression.
Results
Patient population
A total of 9 patients (Table 1) were enrolled in the protocol: 6 males and 3 females from 2012 to 2015. Median age at time of enrollment was 64 years old (range, 33–77 years). Five patients had solitary lesions and 4 patients had multiple lesions. In patient 4, 2 large lesions were adjacent and overlapping and were included in the same field for treatment, all others had separate fields and isocenters. The mean GTV maximum planar diameter was 3.0±0.7 cm (mean volume 13.8±14.8 cc).
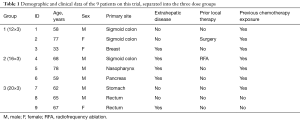
Full table
Toxicity
There were 2 patients with grade 1 toxicity (Table 2). Laboratory evaluation showed no elevation of liver enzymes or bilirubin, no depression of hemoglobin, or platelets in any of the other patients. Patient 5 died within the 90-day period from rapid progression with widely metastatic disease, specifically in the lung.
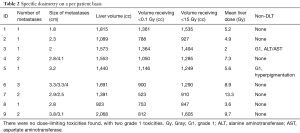
Full table
No late toxicities, other than persistence of the hyperpigmentation, were appreciated. Importantly, there were no cases of DLT or RILD.
Normal tissue dosimetry
The median volume of liver receiving less than 15 was 1,285 cc (V<15 at least 700 cc, IQR 918–1,514 cc) despite several multiple isocenter therapies. Other metrics of liver dosimetry were a median V27 of 11% (V27 <30%, IQR 6–14%) and median V24 of 11.7% (V24 <50%, IQR 6.3–15) respectively.
There were 3 patients receiving any dose to the kidneys with a minimum V<15 of 83.4% (V<15 ≥67%). Maximum dose to stomach/small intestine was 16.6 (maximum dose <30).
Chest wall maximum doses had a median of 29.4 (maximum dose <30, IQR 16.5–34). Median heart maximum dose was 0 (IQR 0–3.4). The median spinal cord maximum was 0 (maximum dose <18, IQR 0–15.7).
Clinical outcomes
Three patients are still alive at the time of this report, with median follow-up and survival of 2.1 years (IQR 0.5–2.2 years, range, 0.2–3.4 years). Two out of 3 patients treated in the low dose cohort had in-field recurrence at 1.4 and 0.7 years which were successfully retreated. To date there are no local failures in the intermediate or high dose cohorts.
Discussion
This is the first reported phase I study of Proton SBRT for liver metastases. Results indicate that a Proton SBRT dose of at least 60 in three fractions may be safely administered to patients. Doses were escalated according to standard phase I design, from 36 to 60 in three fractions in increments of 12 per cohort. There were only 2 episodes of grade 1 toxicity; therefore, the MTD was not reached up to the predefined upper limit of 60.
Historically, local therapy in the context of metastatic disease was rarely considered. However, more recently advances in systemic treatment, surgery and locally ablative techniques have provided promising data suggesting subgroups of patients that may benefit from local therapy of metastatic disease, particularly for oligometastatic disease.
Local surgical therapy of liver metastases has shown significant benefit, including overall survival benefit in select patients (12,13). Unfortunately, a significant majority of patients with liver metastases are not surgical candidates leading to a need for other forms of effective therapy. Other procedures for local therapy include radiofrequency ablation (whether percutaneously or intraoperatively), Yittrium-90, and transarterial chemoembolization (TACE).
Traditionally, radiation has not had a significant role in the treatment of liver tumors due to the relative radiosensitivity of the liver. However, with improved technology (i.e., Intensity Modulated Radiotherapy, improved tumor motion control and onboard imaging), SBRT has been demonstrated to be an effective, safe option for local ablation of tumors. This makes radiation an effective option for these patients with minimal impact on quality of life.
Several pitfalls arise with X-ray based treatment: most prominent issues with regards to liver dose constraints. Liver metastases quite frequently appear in separate lobes of the liver, and out-of-field liver recurrences can occur in more than half of patients (6). Treatment of out-of-field liver recurrences are often either not feasible or require dose reduction to meet dose constraints. In addition, multi-isocentric plans to treat liver metastases that are spread out are typically avoided due to liver dose. However, several series have demonstrated significant decrease in local control with lower BED treatments (3).
Proton therapy offers a distinct advantage in dosimetry. The Bragg Peak effect is utilized which spares significant volumes of the liver from low dose exit radiation. This has allowed us to safely and effectively treat patients with multiple subsequent courses off trial. A metric to describe the feasibility of subsequent courses has not been reported and is conceptually challenging.
In Table 2, the volume of liver receiving no radiation is listed as a possible surrogate marker to imply the feasibility of subsequent courses. A median of 900 cc (IQR 771–1,253 cc) of liver were untouched by radiation in our study patients. As the dose constraint for liver SBRT is for greater than 700 cc to receive <15, this leaves significant liver reserve.
Only one other report of Proton SBRT for liver metastases has been published (9). In this phase II report, doses were individualized based on effective volume of liver irradiated (Veff) with doses ranging from 30 to 50 in 5 fractions (BED 48–100, α/β ratio 10). They reported low toxicity, with no patients having grade 3 or higher toxicity. They noted a relatively low local control rate of 72% at 1 year, indicating that low BED may lead to decreased local control. Our highest dose cohort of 20×3 fractions has a BED of 180 with α/β ratio 10, and we hope this can improve local control.
Conclusions
In conclusion, results of our phase I trial for limited liver metastases have shown 20×3 fractions with Proton SBRT to be a well-tolerated dose with no incidence of DLT. A phase II trial using this dose to assess efficacy is ongoing.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by institutional review board of Loma Linda University Medical Center (IRB# 5120022) and informed consent was taken from all the patients.
References
- Aitken KL, Hawkins MA. Stereotactic body radiotherapy for liver metastases. Clin Oncol (R Coll Radiol) 2015;27:307-15. [Crossref] [PubMed]
- Goodman BD, Mannina EM, Althouse SK, et al. Long-term safety and efficacy of stereotactic body radiation therapy for hepatic oligometastases. Pract Radiat Oncol 2016;6:86-95. [Crossref] [PubMed]
- Ohri N, Tomé WA, Méndez Romero A, et al. Local Control After Stereotactic Body Radiation Therapy for Liver Tumors. Int J Radiat Oncol Biol Phys 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Rusthoven KE, Kavanagh BD, Cardenes H, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol 2009;27:1572-8. [Crossref] [PubMed]
- Radiofrequency Ablation Versus Stereotactic Radiotherapy in Colorectal Liver Metastases. Available online: https://clinicaltrials.gov/ct2/show/NCT01233544
- Joo JH, Park JH, Kim JC, et al. Local control outcomes using stereotactic body radiation therapy for liver metastases from colorectal cancer. Int J Radiat Oncol Biol Phys 2017;99:876-83. [Crossref] [PubMed]
- Ling TC, Kang JI, Bush DA, et al. Proton therapy for hepatocellular carcinoma. Chin J Cancer Res 2012;24:361-7. [Crossref] [PubMed]
- Wang X, Krishnan S, Zhang X, et al. Proton radiotherapy for liver tumors: dosimetric advantages over photon plans. Med Dosim 2008;33:259-67. [Crossref] [PubMed]
- Hong TS, Wo JY, Borger DR, et al. Phase II Study of Proton-Based Stereotactic Body Radiation Therapy for Liver Metastases: Importance of Tumor Genotype. J Natl Cancer Inst 2017.109. [PubMed]
- Wroe AJ, Bush DA, Schulte RW, et al. Clinical immobilization techniques for proton therapy. Technol Cancer Res Treat 2015;14:71-9. [Crossref] [PubMed]
- Michel R, Françoise I, Laure P, et al. Dose to organ at risk and dose prescription in liver SBRT. Rep Pract Oncol Radiother 2017;22:96-102. [Crossref] [PubMed]
- Chua TC, Saxena A, Liauw W, et al. Hepatic resection for metastatic breast cancer: a systematic review. Eur J Cancer 2011;47:2282-90. [Crossref] [PubMed]
- Morris EJ, Forman D, Thomas JD, et al. Surgical management and outcomes of colorectal cancer liver metastases. Br J Surg 2010;97:1110-8. [Crossref] [PubMed]


