
Outcomes of endoscopic submucosal dissection in esophageal adenocarcinoma staged T1bN0 by endoscopic ultrasound in non-surgical patients
Introduction
Endoscopic resection (ER) is considered as a preferred treatment for early esophageal adenocarcinoma (EAC) and has been shown to be curative in certain submucosal cancers which are confined to the outer third of the submucosa (sm1). ER can be accomplished by endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD). ESD is associated with higher en bloc and curative resection rates and it may be preferred over EMR in selected cases such as lesions larger than 20 mm, poorly lifting tumors, and lesions at risk for submucosal invasion (1). In this study, we present the outcomes in patients diagnosed as T1bN0 EAC on EUS who underwent ESD by one of the authors (MR Sanaka).
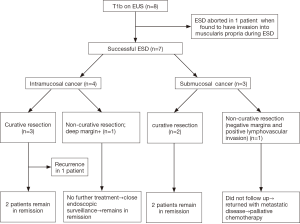
All the cases were discussed at a multi-disciplinary tumor board meeting involving representatives from medical oncology, radiation oncology, thoracic surgery and gastroenterology where ESD was deemed as an appropriate treatment option. Patients were explained the non-negligible risk of lymph node involvement and need for additional surgery, radiation or chemotherapy based on the results of the ER. Detailed flowchart is outlined in Figure 1.
ESD procedure
All ESD procedures were performed by a single operator (MR Sanaka) under general anesthesia and carbon dioxide insufflation through the endoscope. Standard ESD technique of circumferential marking, incision and sub-mucosal dissection was performed. Methylene blue stained Voluven was used as sub-mucosal injection solution. Devices used were Dual knife, IT Nano knife and Coag-graspers (Olympus USA). All patients were admitted post-procedure for observation. An esophagram was performed on post-operative day one and patients were started on clear liquid diet if no leak was detected. Diet was advanced and if patients were stable, they were discharged home on oral proton pump inhibitors for at least 2 months.
Definition of outcomes
En bloc resection: defined as excision of the targeted lesion in a single specimen.
Piecemeal resection: defined as excision of the targeted lesion in more than one piece.
R0 resection: defined as histologically complete resection with deep and lateral margins negative for malignancy irrespective of the presence of high-grade dysplasia (HGD) or intestinal metaplasia (IM).
R1 resection: defined as histologically incomplete resection with positive deep or lateral margins.
Curative resection: patients with R0 resection, with well to moderately differentiated histology, absence of LVI and absence of invasion beyond superficial submucosa (sm1) were considered to have curative resection.
Results
Eight patients met the inclusion criteria during the study period (male =5; female =3). All patients were Caucasians with an average age of 70.5 years (range, 53–84 years). Seven patients were classified as American Society of Anesthesiology (ASA) classification III and one patient was ASA class IV. EUS staging was T1bN0 and Paris classification was 0–Is + IIa in 4 patients, 0–IIa+IIb in 3 patients and 0–IIa + IIc in 1 patient. ESD was successfully completed in 7 patients and aborted in 1 patient, in whom the tumor was noted to invade the muscularis propria layer (T2) at the time of ESD. Average procedure duration (time from endoscope in to endoscope out) was 190.8 minutes (range, 105–352 minutes). Average diameter of the resected specimen was 32.3 mm (range, 16–50 mm) and average diameter of the resected tumor was 15.14 mm (range, 4–40 mm).
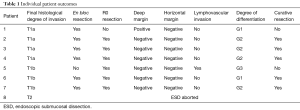
En bloc, R0 and curative resection rates were 86% (6/7), 86% (6/7) and 71% (5/7) patients respectively. Focal positive deep tumor margin and LVI were present in one patient each. On histopathological assessment, 57% (4/7) tumors were intra-mucosal (T1a), whereas only 43% (3/7) had submucosal invasion (T1b). Well-differentiated, moderately differentiated and poorly differentiated tumor were present in 29% (2/7), 57% (4/7) and 14% (1/7) cases respectively. Average length of stay (LOS) was 1.8 days (range, 1–3 days). There were no intra-procedural adverse events. One patient (1/7: 14%) developed an esophageal stricture in the post-operative period requiring esophageal balloon dilatation. Of the 4 patients with only mucosal invasion (T1a) on final histology, 3 patients had curative resection and 1 patient had non-curative resection due to focally positive deep margin. Out of 3 patients with curative resection, 1 patient developed recurrence of cancer during follow-up period and underwent radiation therapy and is currently in remission. The fourth patient with T1a tumor with non-curative resection was noted to be negative for malignancy on follow-up EGD with biopsies and is currently tumor-free and under close endoscopic surveillance. Of the 3 patients with submucosal invasion (T1b, sm1), 2 patients had curative resection and 1 patient had non-curative resection due to presence of LVI. Both the patients with curative resection remain in remission on endoscopic follow up. One Patient with non-curative resection was subsequently noted to have distant metastasis and is undergoing palliative chemotherapy. Average follow-up duration was 10 months (range, 3–15 months) and 71% (5/7) patients remain in clinical remission at the last follow up. Individual patient outcomes are shown in Table 1.
Full table
Discussion
Endoscopic therapy for EAC is gaining gradual acceptance in the United States. ER is a less invasive alternative to esophagectomy in patients with mucosal cancer associated with Barrett’s esophagus (BE). Recent studies have shown that certain lesions with superficial submucosal invasion (≤500 µm, sm1) and low risk features (well or moderately differentiated tumor (G1 –2), without LVI (L0 and V0), and smaller size (<3 cm) may also be amenable to ER, as they harbor low risk of lymph node metastasis (2-4). EMR is the treatment of choice for resection of visible lesions in patients with BE. ESD enables en bloc resection of the lesions regardless of the tumor size, allowing for a detailed histopathological analysis, high curative rates and low rates of local recurrences and may be preferred for larger lesions (1,5). ESD is extensively performed in Japan and other eastern countries and is considered the first line treatment for the superficial squamous cell esophageal cancer (SQEC) (1). Studies from eastern hemisphere on ESD for EAC have reported high rates of en bloc resection (81–100%), R0 resection (38% to 97%), curative resection (64% to 86%) and mean procedure times of 70–145 minutes (3-9). In our study, the en bloc resection rate, R0 resection rate and curative resection rate were 86%, 86% and 71% respectively, which are comparable to above studies. However, the average procedure time was much longer in our study (190 minutes), since ESD was only recently started in our institution and it is associated with significant learning curve.
EUS was reported to be inaccurate to differentiate between mucosal (T1a) and submucosal tumors (T1b) (10-14). Recent studies indicate that EUS under-staged 15–25% of cases and over-staged 4–12% cases, as compared to staging by EMR (15,16). Even in our study, EUS accurately staged T1b tumor in only 37.5% (3/8), over-staged 50% (4/8) cases and under-staged in 12.5% (1/8) cases. Relying on EUS as a “T” staging method in early carcinomas might result in some potentially endoscopically resectable tumors being subjected to esophagectomy. Therefore, EUS is not routinely recommended by some experts prior to EMR/ESD for superficial cancers. On the contrary, EUS provides more accurate staging for advanced ECs and it is better than PET and CT for evaluation of both T and N staging (17). Therefore, EUS may be used for nodal staging and staging of high risk lesions as they have a greater risk of invasiveness.
Our study has some limitations. This is a retrospective study performed by a single operator at a single center with a very small number of patients. Even the ESD procedures performed during the early part of the learning curve were also included (within initial 25 ESD cases). This could have impacted the ESD outcomes including procedure length. Currently ESD is performed in only a few select centers in the US and our study findings may not be generalizable. Also, our outcomes are reported based on a short-term follow up and long-term outcomes are not known. Strengths of this study include a thorough and complete multi-disciplinary evaluation and careful selection of patients. This is one of the very few reports on outcomes of ESD in T1b EAC, especially from the US.
Conclusions
In conclusion, ESD is safe and is associated with favorable outcomes in EAC staged T1bN0 by EUS in poor surgical candidates. In addition, ESD led to a more accurate histological staging than EUS. In patients with early EAC, definitive treatment decisions should not be made solely on the basis of EUS because of its inability to accurately differentiate between mucosal and sub-mucosal involvement. After confirming the absence of loco-regional and distant metastasis, ESD with or without additional treatments may be considered as an alternative to definitive CRT in T1b lesions among poor surgical candidates. ESD would be both diagnostic and therapeutic in some cases and helps to determine further course of treatment. Prospective trials are warranted in western world to assess the efficacy of ESD plus/versus CRT in non-surgical candidates with invasive T1b EAC.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2015;47:829-54. [Crossref] [PubMed]
- Manner H, May A, Pech O, et al. Early Barrett's carcinoma with "low-risk" submucosal invasion: long-term results of endoscopic resection with a curative intent. Am J Gastroenterol 2008;103:2589-97. [Crossref] [PubMed]
- Hirasawa K, Kokawa A, Oka H, et al. Superficial adenocarcinoma of the esophagogastric junction: long-term results of endoscopic submucosal dissection. Gastrointest Endosc 2010;72:960-6. [Crossref] [PubMed]
- Yoshinaga S, Gotoda T, Kusano C, et al. Clinical impact of endoscopic submucosal dissection for superficial adenocarcinoma located at the esophagogastric junction. Gastrointest Endosc 2008;67:202-9. [Crossref] [PubMed]
- Ono S, Fujishiro M, Koike K. Endoscopic submucosal dissection for superficial esophageal neoplasms. World J Gastrointest Endosc 2012;4:162-6. [Crossref] [PubMed]
- Hoteya S, Matsui A, Iizuka T, et al. Comparison of the clinicopathological characteristics and results of endoscopic submucosal dissection for esophagogastric junction and non-junctional cancers. Digestion 2013;87:29-33. [Crossref] [PubMed]
- Neuhaus H, Terheggen G, Rutz EM, et al. Endoscopic submucosal dissection plus radiofrequency ablation of neoplastic Barrett's esophagus. Endoscopy 2012;44:1105-13. [Crossref] [PubMed]
- Kakushima N, Yahagi N, Fujishiro M, et al. Efficacy and safety of endoscopic submucosal dissection for tumors of the esophagogastric junction. Endoscopy 2006;38:170-4. [Crossref] [PubMed]
- Chevaux JB, Piessevaux H, Jouret-Mourin A, et al. Clinical outcome in patients treated with endoscopic submucosal dissection for superficial Barrett's neoplasia. Endoscopy 2015;47:103-12. [PubMed]
- NCCN Guidelines Version 4 2017. Esophageal and Esophagogastric Junction Cancers. Available online: https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf
- Pech O, Günter E, Dusemund F, et al. Value of high-frequency miniprobes and conventional radial endoscopic ultrasound in the staging of early Barrett's carcinoma. Endoscopy 2010;42:98-103. [Crossref] [PubMed]
- Thosani N, Singh H, Kapadia A, et al. Diagnostic accuracy of EUS in differentiating mucosal versus submucosal invasion of superficial esophageal cancers: a systematic review and meta-analysis. Gastrointest Endosc 2012;75:242-53. [Crossref] [PubMed]
- Young PE, Gentry AB, Acosta RD, et al. Endoscopic ultrasound does not accurately stage early adenocarcinoma or high-grade dysplasia of the esophagus. Clin Gastroenterol Hepatol 2010;8:1037-41. [Crossref] [PubMed]
- Rampado S, Bocus P, Battaglia G, et al. Endoscopic ultrasound: accuracy in staging superficial carcinomas of the esophagus. Ann Thorac Surg 2008;85:251-6. [Crossref] [PubMed]
- May A, Günter E, Roth F, et al. Accuracy of staging in early oesophageal cancer using high resolution endoscopy and high resolution endosonography: a comparative, prospective, and blinded trial. Gut 2004;53:634-40. [Crossref] [PubMed]
- Larghi A, Lightdale CJ, Memeo L, et al. EUS followed by EMR for staging of high-grade dysplasia and early cancer in Barrett's esophagus. Gastrointest Endosc 2005;62:16-23. [Crossref] [PubMed]
- Berry MF. Esophageal cancer: staging system and guidelines for staging and treatment. J Thorac Dis 2014;6 Suppl 3:S289-97. [PubMed]


