|
Review Article
Nanovector-based therapies in advanced pancreatic cancer
Chang-Sung Tsai1,2, John W. Park3, Li-Tzong Chen1,2,4
1National Institute of Cancer Research, National Health Research Institutes, Tainan, Taiwan; 2Department of Internal Medicine, National Cheng Kung
University Hospital, Tainan, Taiwan; 3Helen Diller Family Comprehensive Cancer Center, UCSF, San Francisco, California, USA; 4Department of Internal
Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan
Corresponding to: John W. Park, Professor. UCSF Helen Diller Family Comprehensive
Cancer Center, 1600 Divisadero St., 2nd Fl., San Francisco, CA
94115-1710. Tel: 415-502-3844; Fax: 415-353-9592. E-mail: jpark@cc.ucsf.edu.
Li-Tzong Chen, Professor. National Institute of Cancer Research, National
Health Research Institutes, No 367, Sheng-Li Road, Tainan 70456, Taiwan.
Tel: +886-6-208 3422; Fax: +886-6-208 3427. Email: leochen@nhri.org.tw.
|
|
Abstract
Systemic therapy for advanced pancreatic cancer has been largely disappointing owing to the unfavorable pharmacokinetic
profile and poor penetration of current chemotherapeutic agents ,as well as the fragile patient population with
compromised tolerance to toxic chemotherapies. Nanovectors can provide passive drug delivery through abnormal tumor
neo-vasculature microanatomy or active targeting via binding to receptors or macromolecules associated with the
tumor. In such a manner, nanovector-based therapy may not only modulate the pharmacokinetics and therapeutic index
of chemotherapeutic agents but also provide new treatment options in patients with advanced pancreatic cancer. In this
article, we present the rationale and currently available clinical results of nanovector-based therapies to highlight the potential
use of this class of agent in patients with advanced pancreatic cancer.
Key words
nanovector; pancreatic cancer; liposome; PEP02; nab-paclitaxel; EndoTAG-1; nanoplatin; platinum; CPT-11
J Gastrointest Oncol 2011; 2: 185-194. DOI: 10.3978/j.issn.2078-6891.2011.034
|
|
Introduction
Pancreatic cancer is one of the most detrimental
malignancies and the fourth most common cause of
cancer-related death in the United Stated. There were
43,140 newly diagnosed cases and 36,800 deaths in
2010 ( 1). Early detection is uncommon with no more
than 15–20% of the patients being amenable for curative
intent surgery at the time of diagnosis. Gemcitabine
either alone or in combination with erlotinib are the
only approved treatments for patients with advanced
pancreatic cancer, of whom the overall survival time is
generally around 6 months ( 2-5). Recently, Conroy et
al showed that a gemcitabine-free triplet chemotherapy,
FOLFIR INOX regimen consisting of oxaliplatin,
irinotecan and infusional 5-FU/leucovor in, could achieve significantly better tumor response rate,
progression-free survival and overall survival than
gemcitabine monotherapy in patients with metastatic
pancreatic cancer in a randomization phase III trial ( 6, 7).
However, the application of either doublet of triplet
combination chemotherapy in patients with advanced
pancreatic cancer is often hindered by their toxicity and
the performance status of the patients. New treatment strategies are mandatory to improve
the therapeutic outcomes of patients with advanced
pancreatic cancer. Recently, two major potential new
approaches are emerging that may have the chance to
change our practice in treating advanced pancreatic
cancer. The first one is molecular targeted agent targeting
on dysregulated signaling pathway and the second is
the use of nanovector drug delivery system to provide
‘passive” or “active” targeting drug delivery thus to
modulate the pharmacokinetics and therapeutic index of
chemotherapeutic agents in pancreatic cancer ( 8). This review will focus on the selective nanovector
treatments in pancreatic cancer, especially those with
available clinical data , including albumin-bound
nanoparticles, liposome-encapsulation nanoparticle,
cationic liposomal nanoparticle, polymeric micellar agents,
and a non-replicating, retroviral vector delivered gene
therapy construct.
|
|
Albumin-bound Nanoparticle Paclitaxel (Nab-paclitaxel)
Albumin is a particular vehicle for drug delivery in oncology
because it is a natural carrier of hydrophobic molecules
with reversible, noncovalent binding characteristics and
able to enhance the delivery of drug into the extravascular
space through a process of receptor-mediated endothelial
transcytosis. Such process is initiated by the binding of
albumin to an endothelium surface, 60-kDa glycoprotein
(gp60) receptor (albondin), which will then bind with
an intracellular protein (caveolin-1) to result in the
invagination of the endothelium membrane to form
transcytotic vesicles, the caveolae ( 9). The caveolae will
subsequently move across the cytoplasm and release the
albumin and its conjugated compound into the extracellular
space (the peritumoral microenvironment) where the
albumin will bind to SPARC (secreted protein acid and
rich in cysteine), an extracellular matrix albumin-binding
glycoprotein that is structurally and functionally closely
related to gp60, and overexpressed in a variety of cancers,
including breast cancer, gastric cancer and pancreatic
cancer. Nab-paclitaxel (Abraxane®) is a cremophor (CrEL)-free,
albumin-bound, nanoparticle formulation of paclitaxel.
Its CrEL-free formulation permits nab-paclitaxel to be
administered within a shorter infusion period of time
(30 minutes) and without the requirement of routine premedications
for preventing the hypersensitivity reactions in
association with the administration of cremophor solventbased
paclitaxel ( 10). In preclinical study, the transport of
radiolabeled paclitaxel across the endothelial cell monolayer
in vitro, and intratumor paclitaxel accumulation after equal
doses of paclitaxel in vivo were both significantly enhanced
by 4.2-folds (P < 0.0001) and 33% (P < 0.0001), respectively,
for nab-paclitaxel as compared with CrEL-paclitaxel with
an increase 4.2 folds. In addition, endothelial transcytosis
was completely inhibited by inhibitor of gp60/caveolar
transport, methyl ß-cyclodextrin ( 11). These observations
supported that gp60-mediated transcytosis and SPARCaided
sequestration may be an important biological pathway
to target tumor cells by novel albumin-bound therapeutics. In a phase I trial, the maximum tolerated dose (MTD)
of intravenous injection nab-paclitaxel monotherapy,
every 3 weeks in 19 patients with standard therapy-failure
solid tumors was 300 mg/m 2. No acute hypersensitivity
reactions were observed. The most frequent toxicities were
myelo-suppression, sensory neuropathy, nausea/vomiting,
arthralgia and alopecia ( 12). The drug has subsequently
approved for the treatment of metastatic breast cancer after
failure of combination chemotherapy or relapse within
6 months of adjuvant chemotherapy. The commonly used dose/schedule was 260 mg/m 2, 30-min intravenous
injection, every 3 weeks. Because SPARC is f requently overexpressed and
associated with poor clinical outcomes in pancreatic cancer,
Von Hoff et al conducted a phase I/II study to evaluate the
MTD of weekly nab-paclitaxel (100 – 150 mg/m 2/week)
in combination with gemcitabine (1000 mg/m 2/week),
and the therapeutic efficacies of the regimen. Both agents
were given on day 1, 8, and 15 every 28 days ( 13). A total of
67 patients were treated. Despite MTD of nab-paclitaxel
was determined as 125 mg/m 2/week, dose reduction was
required in 30% (6/20), 18% (8/44) and 33% (1/3) of
patients receiving 100 mg/m 2, 125 mg/m 2 and 150 mg/m 2,
respectively. The most common grade 3-4 toxicity at the
MTD dose were fatigue 23%, neutropenia 59% (grade 4 in
23%), thrombocytopenia 20% (grade 4 in 9%) and sensory
neuropathy in 9%. Of the 58 patients whose CT image were
revaluated with RECIST criteria by independent reviewer,
the best tumor response was partial response in 40% and
stable disease in 37%, with an overall disease control rate
of 78%. The median progression-free and overall survival
of the intent-to-treat (N=67) patients were 6.9 months and
10.3 months, respectively; while the survival parameters for
the 44 patients receiving MTD dose were 7.9 months and
not yet reached, respectively. Of 54 patients with available
CA19.9 level, 42 (77.8%) patients had a more than 50%
reduction of CA19.9 level after the treatment ( 14). The
therapeutic efficacy of nab-paclitaxel in combination with
vandetanib, a potent inhibitor of VEGF2, RET and EGFR,
has also been evaluated in a phase I trial with expansion
cohort of patients with pancreatic cancer ( 15). The MTD
of vandetanib in combination with two different schedule
of nab-paclitaxel, either 100 mg/m 2 weekly or 260 mg/
m2 every 3 weeks, was 300 mg daily. Of the 29 enrolled
gemcitabine-refractory pancreatic cancer patients, the best
tumor was partial response in 6 (20.7%) and stable disease
in 10 (34.5%), and the median progression-free survival and
overall survival were 5.3 (95% CI: 3.7 to 7.3) months and
8.2 (95% CI: 6.2 to 11.5) months, respectively. No statistical
significant correlation between SNP (rs1059829 and
rs3210714) of SPARC and clinical outcomes was observed.
|
|
Liposome-based Drugs
A liposome is often a spherical vesicle with a bilayer
membrane whose size typically ranges from ~40 nanometers
to several microns. Because the micro- or nanoparticles
can form spontaneously and are generally easier to prepare
compared to viral-mediated systems, this nontoxic
phospholipid-based drug carrier has become a favorable
drug delivery system for various purposes since the 1970s. However, so-called conventional liposomes are easily bound
with insoluble circulating plasma protein, i.e. opsonins
and lipoproteins, and the complex will be subsequently
eliminated from the circulation by reticuloendothelial cells
system. Stealth liposome technology, with incorporationof
high molecular weight polymers (i.e., polyethylene-glycol
(PEG)) to the liposome surface, can effectively protect the
liposome from circulating protein binding and subsequently
phagocytosis by RER system, and thus improving its plasma
clearance, prolonging the circulation time, and enhancing
drug delivery efficacy.
Besides its characteristic slow-release pharmacokinetic
property, liposome encapsulated drugs can potentially
provide improved tumor localization via the “enhanced
permeability and retention” (EPR) effect. Such agents can
therefore, (i) lower drug elimination to increase systemic
circulation time, (ii) lower maximum plasma concentration
(Cmax) to reduce drug side effects, (iii) enhance tumor
tissue uptake and exposure to the anti-cancer drug; these
principles can in turn yield an improved therapeutic index
for cancer therapy.
Several liposomal formulated cancer drugs have been
evaluated in various cancers, but only a limited number
have been applied to pancreatic cancer.
|
|
Liposomal Doxorubicin
The first liposomal anti-cancer drug approved by the Food
and Drug Administration (FDA) was pegylated liposomal
doxorubicin (Caelyx®/Doxil®) in 1995 for Karposi ’s
sarcoma ( 16-18). It has been subsequently approved for the
treatment of multiple myeloma and recurrent epithelial
ovarian cancer as well. It also has been evaluated for
the treatment of pancreatic cancer in animal xenograft
model and in clinical trials. In a preclinical study, Vagge
et al showed that pegylated liposomal doxorubicin was
significantly more effective in inhibiting the growth
of human pancreatic cancer xenograft in nude mice as
compared to free form doxorubicin ( 19). Using confocal
laser scanning microscopy and microf luorimetry to
quantitate the uptake of intravenously injected doxorubicin
in tumor tissue, the authors found that the content of
doxorubicin in tumor site of animal receiving liposomal
formulated drug was 6 folds or higher compared to free
doxorubicin. Based on the results, Halford et al conducted
a phase II trial to evaluate the therapeutic efficacy of
Caelyx® in 22 chemo-naïve patients with unresectable
pancreatic carcinoma. The dose was escalated from 30
mg/m 2 (in the first two patients) to 50 mg/m 2 intravenous
injection every 3 weeks ( 20). Of the 20 patients received
the treatment, the most common grade 3 toxicity were stomatitis (20%) and nausea (10%), the best tumor response
was stable diseases in 6 (30%), and the median overall
survival was 3.2 months with one year survival rate of 10%.
These finding excluded the use of Caelyx® monotherapy in
the treatment of advanced pancreatic cancer. The combination of Caelyx® with infusional 5-FU/
leucovorin and mitomycin-C has been evaluated in a phase
I trial in patients with upper gastrointestinal cancer. In
that study, escalating dose of Caelyx® (15 – 35 mg/m 2) day
1 and 29 in combination with weekly 24-hour infusion of
5-FU and leucovorin (2,000 and 500 mg/m 2, respectively)
for 6 weeks, and mitomycin-C 7 mg/m 2 day 8 and 36,
every 8 weeks as one cycle. The most common grade 3-4
toxicities were nausea/vomiting (29%), diarrhea (18%)
and leucopenia (12%). Of the 14 accruals with pre-treated
pancreatic cancer, the best tumor response was partial
response in one and minor response in 2, and the overall
survival after the study treatment was 6.5 months ( 21).
|
|
Liposomal Platinum
Platinum is one of the most active and wildly used anticancer
agents in the world, including in combination
with gemcitabine to treat non-small cell lung cancer
and pancreatic cancer. Although each single trial had
failed to demonstrate the superiority of gemcitabine/
platinum combination over gemcitabine single agent in
the prolongation of the survival in patients with advanced
pancreatic cancer, however, the sur v iva l benef it of
gemcitabine/platinum doublets was demonstrated in a
pooled, meta-analysis survival with a hazard ratio of 0.81, p
= 0.031 ( 22). It is also well known that the use of cisplatin is frequently
limited by its nephrotoxicity, peripheral sensory neuropathy,
ototoxicity and the aggravation of hematological toxicity
while in combination with other cytotoxic agents. Therefore,
several liposomal formulations of cisplatin have been
developed aiming to reduce its toxicity profile and hopefully
to enhance it activity. Based on previous experience of
gemcitabine/cisplatin combination and the result of metaanalysis,
several liposomal formulated cisplatin have been
evaluated in patients with pancreatic cancer.
L ipoplatin is one of the pegylated liposome
cisplatin, whose nanoparticulate liposomes are reversemi
scelles , composed of dipa lmitoyl phosphat idyl
glycerol (DPPG), soy phosphatidyl choline (SPC-3),
cholesterol and methoxy- polyethylene glycol-distearoyl
phosphatidylethanolamine (mPEG2000-DSPE). Lipoplatin
exhibits the fundamental pharmacologic characteristics
of pegylated liposomal agents, for example, protecting
from the engulfment of reticuloendotheralial system to prolong circulating time, and extravasating from the
fenestrate between endothelial cells of tumor vasculature
to preferentially localize in per-tumor interstitial tissue and
uptake by tumor cells. The anionic, fusogenic nature of the
DPPG lipids enables lipoplatin to cross cell membranes
more easily than native cisplatin. In addition, with
intraperitoneal injection of a “sheath” liposomes wrapped
reporter β-galactosidase gene, which had same structure
like lipoplatin, into human tumor bearing nude mice,
Boulikas et al were able to demonstrate the preferential
expression of the reporter gene in the tumor and the
tumor neo-vasculature. The findings indicate the potential
antiangiogenic activity of the lipoplatin ( 23). In phase I trial of lipoplatin monotherapy, the drug
was diluted in 5% glucose water and administered as 8
hour intravenous infusion every 14 days. The dose was
escalated from 25 mg/m 2 to 125 mg/m 2. Even at the
targeted dose of 125 mg/m 2, only grade 1-2 gastrointestinal
and hematological toxicities were observed, but neither
nephrotoxicity nor neuropathy. Higher doses, 200, 250
and 300 mg/m 2, were also tested in one each patient,
respectively. The half-life of lipoplatin was estimated
ranging from 60 – 117 hours. Of the 27 accruals (19 with
pretreated, advanced pancreatic cancer) in this phase I trial,
the objective tumor response rate and disease control rate
were 11.1% and 63.0%, respectively. Based on the exciting
results, the drug has been further tested in combination
with gemcitabine in non-small cell lung cancer and
pancreatic cancer patient cohorts ( 24). In a phase I/II study, Stathopoulos GP et al evaluated the
maximum tolerated dose of lipoplatin in combination with
gemcitabine in patients with previously treated advanced
pancreatic cancer ( 25). Lipoplatin was given as an 8-hour
infusion followed by 60 minutes infusion of 1,000 mg/m 2
of gemcitabine at day 1 and 15 every 28 days. The dose of
lipoplatin was stepwise escalated from 25 mg/m 2 to 125
mg/m 2. Of the 24 enrolled patients, two of four patients at
125 mg/m 2 experienced grade 3-4 neutropenia. Therefore,
the MTD of lipoplatin in this combination was determined
to be 100 mg/m 2. In this dose escalating study, there were
two (8.3%) partial responders and 14 (58.3%) disease
stabilizers, and the median overall survival was 4 month.
Further randomized phase II/III trial against gemcitabine
monotherapy is under evaluation. Liposome-entrapped cis-bisneodecanoato-trans-R,R-
1,2-diaminocyclohexane (DACH) platinum(II) (L-NDDP,
Aroplatin™) is a lipophilic cisplatin analog that has been
formulated in relatively large-size multi-lamellar liposomes
measuring from 1 to 3 μm in diameter. L-NDDP has been
demonstrated to be non–cross-resistant with cisplatin in
cisplatin-resistant Lovo DDP 3.0 (human colon cancer cells) and L1210/PPD (human leukemia cells) both in
vitro and in vivo models. In a phase I study, L-NDDP was
given intravenously once every 4 weeks, ranging from
7.5 mg/m 2 to 390 mg/m 2 ( 26). The infusion rate was set
at 4 mg NDDP per minute for all cases. In this particular
study, intra-patient dose escalation was allowed. Grade
1-2 nausea/vomiting, diarrhea and fever were frequently
observed in patients receiving 100 mg/m 2 or higher dose
of L-NDDP. Six out of the 10 patients who had 390 mg/m 2
experienced grade 4 hematological toxicities manifesting as
thrombocytopenia, granulocytopenia or both. The MTD of
intravenous L-NDDP every 4 weeks was determined as 300
mg/m 2. In 2004, Aronex Pharmaceuticals had registered a
phase I/II study of L-NDDP and gemcitabine combination
in patients with advanced pancreatic cancer resistant
to standard therapy in a public clinical trial registration
website, the clinicaltrials.gov, with an indentifier of
NCT00081549. Unfortunately, the latest trial information
was updated in June 2005, and no further publication on
this trial can be found.
|
|
Liposomal Irinotecan (Nanoliposomal CPT-11,
PEP02, MM-398)
Irinotecan hydrochloride (CPT-11) is a water-soluble
semi-synthetic derivative of camptothecin targeting
topoisomerase I, and has been an approved agent for the
treatment of metastatic colorectal cancer worldwide, and
also for gastric cancer (Japan and Korea), non-small cell
lung cancer, small cell lung cancer, cervical cancer, and non-
Hodgkin’s lymphoma in Japan. In pancreatic cancer, earlier
trial showed that combination of gemcitabine and CPT-11
did not provide any survival benefit over gemcitabine
monotherapy in patients with advanced pancreatic
cancer, and thus CPT-11 has not been considered to be a
clinically useful drug in this disease. However, in the recent
PRODIGE 4/ACCORD 11 trial, Conroy et al demonstrated
that a gemcitabine-free, CPT-11-containing regimen,
FOLFIRINOX (CPT-11, oxaliplatin plus intermittent
infusion of 5-FU/leucovorin), provided significantly better
objective tumor response rate, progression-free survival
and overall survival versus gemcitabine monotherapy in
patients with metastatic pancreatic cancer. Notable and
not unexpectedly, this triplet regimen is associated with
significant hematologic toxicity including higher rates of
grade-3/4 febrile neutropenia. The results of the PRODIGE/
ACCORD 11 trial have revived interest in CPT-11-based
therapy in advanced pancreatic cancer ( 6, 7). Although the original CPT-11 drug is now of interest
in pancreatic cancer management, potentially superior
versions incorporating drug delivery technologies offer a next generation approach. CPT-11 exhibits well-known
pharmacologic liabilities and signif icant associated
toxicities, which in turn make it an obvious candidate for
drug delivery strategies The camptothecins exist in a pHdependent
equilibrium between an inactive carboxylate
form (predominant at neutral-to-basic pH) and an active
lactone form (predominant under acidic conditions);
hence, intravenous injection of free CPT-11 results in rapid
inactivation as well as clearance. Furthermore, CPT-11
is largely a prodrug which is converted into the much
more potent metabolite SN-38. Hepatic activation and
hepatobiliary excretion of SN-38 result in substantial risk of
GI injury, especially in individuals having impaired SN-38
glucuronidation. These metabolic conversions contribute
to notable heterogeneities in both efficacy and toxicity, and
ultimately to a rather narrow therapeutic index. The concept
of nanoparticle delivery of CPT-11 is thus very attractive
based on potential advantages including: overcoming
solubility limitations of the camptothecins; protecting
drug in the active lactone configuration; chaperoning drug
away from sites of toxicity such as the GI tract; prolonging
circulation time and increasing tumor accumulation via
the enhanced permeability and retention (EPR) effect; and
providing sustained release and prolonged tumor exposure.
To realize the potential advantages of nanoparticle
delivery, a novel liposome-based construct termed
“nanoliposomal CPT-11 (nLs-CPT-11)” was developed,
which encapsulates CPT-11 with unprecedented efficiency
and stability ( 27). PK studies showed long circulation times
for the carrier and undetectable drug release in plasma.
Furthermore, nanoliposomal CPT-11 provides protection of
drug in its active lactone form within the liposome aqueous
interior, preventing its hydrolysis as well as premature
conversion to the potent and toxigenic metabolite, SN-38.
This contrasts markedly with free CPT-11, which is rapidly
cleared from circulation, is subject to immediate hydrolysis
of the lactone ring, and is also conver ted to SN-38
contributing to its dose-limiting GI toxicity. In a series of preclinical studies, nanoliposomal
CPT-11 demonstrated significantly superior efficacy when
compared to free CPT-11 at the same or higher dose,
including frequent cures in some models. The superiority of
nanoliposomal CPT-11 over free CPT-11 has been observed
in different tumor models including colorectal, gastric,
breast, cervical, glioma, pancreatic and lung cancer models.
In addition to superior efficacy, nanoliposomal CPT-11 has
shown a more favorable pharmacologic profile and reduced
toxicity in multiple preclinical models.
In order to evaluate this novel agent as a potential therapy
for pancreatic cancer, a bioluminescence-based orthotopic
xenograft model of pancreas cancer was developed ( 28). COLO357, a human pancreatic cell line, was passaged
multiple times in vivo to generate the subline L3.6pl. This
cell line was then modified by lentiviral transduction
(L3.6pl-T) to express firefly luciferase. L3.6pl-T cells were
implanted during open surgery directly into the pancreas
of a nude mouse to form an orthotopic tumor xenograft.
Therapeutic studies in this model compared nanoliposomal
CPT-11 versus free drug at the equivalent dose, along with
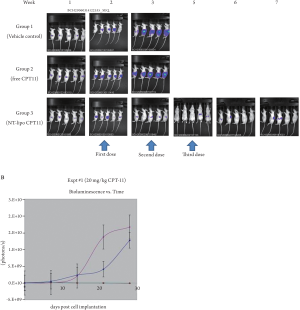
vehicle control ( Figure 1). All treatments were administered
intravenously by tail vein beginning at 7 days post-tumor
implantation and continued weekly for a total of 3 planned
treatments. At 20 mg/kg, free CPT-11 showed some tumor
growth inhibition, but all mice required euthanization
after 2 doses due to massive tumor progression. In contrast,
nanoliposomal CPT-11 at the equivalent 20 mg/kg dose
showed potent antitumor activity, including complete
tumor inhibition during the entire post-treatment period.
Systemic toxicity was not observed with any treatment.
These studies indicated that nanoparticle- mediated delivery
via nanoliposomal CPT-11 greatly enhances antitumor
efficacy in the COLO357/L3.6pI-T orthotopic pancreatic
xenograft model. In the first-in-human phase I trial, patients with
standard therapy-failure solid tumor were enrolled to
determine the maximum tolerated dose, safety profile and
pharmacokinetics of nanoliposomal CPT-11 (formerly
PEP02, PharmaEngine, Inc., Taiwan, and now under the
designation of MM-398, Merrimack Pharmaceuticals, Inc,
USA). The drug was delivered intravenously for 90 minutes,
once every 3 weeks, with starting dose of 60 mg/m 2. The
maximum tolerated dose was 120 mg/m 2. Two patients
achieved partial response including cervical cancer in
one and pancreatic cancer in one ( 29). The observation
was further extended in a phase I trial for nanoliposomal
CPT-11in combination with weekly 24-hour infusion of
high-dose 5-FU/LV (HDFL). In the two phase I trials, 7
pancreatic cancer patients who failed gemcitabine/HDFL
+/- platinum had received PEP02 with or without HDFL.
The best response was partial response in one, stable
disease in 4 and progressive disease in 2, which indicated
a potential activity of PEP02 in treating gemcitabinerefractory
advanced pancreatic cancer. Based on these
clinical observations and preclinical results, clinical
testing of nanoliposomal CPT-11 was pursued in patients
with gemcitabine-based chemotherapy failure advanced
pancreatic cancer in an international phase II trial with the
target of the primary end-point of 3-month overall survival
rate (OS 3-month) = 65%. The results have been presented at the
2011 ASCO meeting ( 30). Of the 40 treated patients, more
than three fourths had failed to first-line gemcitabine-based
doublet or triplet chemotherapy. Mean cycle of treatment was 5.4 (range, 1 – 26) cycles. The most common G3/4
toxicities were: neutropenia (30%), leucopenia (22.5%),
anemia (15%), diarrhea (7.5%), and fatigue (7.5%). Dose
modification due to adverse events was required in 10 (25%)
patients. The best tumor response rate was partial response
in 7.5% and stable disease in 40% (overall disease control
rate of 47.5%). The overall survival was 5.2 months with
a 3-month and 6-month survival rate of 75% and 42.5%,
respectively. The results highlight the feasibility and activity
of nanoliposomal CPT-11 in previously heavily treated
patients with gemcitabine-refractory advanced pancreatic
cancer, which deserves further exploration.
|
|
Cationic Liposome Encapsulated Paclitaxel
(EndoTAG™-1)
Tumor angiogenesis, the formation of neovasculature
from pre-existed peri-tumor vessels, is a crucial process
in supporting the development and growth of tumor
mass, and the dissemination of tumor metastases. Tumor
angiogenesis is mainly triggered by growth factors that
are secreted by tumor cells per se and/or by miscellaneous
types of cell within the microenvironment, for example,
tumor associated macrophages or fibroblasts. Tumor vessels
are often dilated and torturous, and characterized by large
inter-endothelial cell gap (up to 100 – 600 nm versus < 6
nm in normal vessels), aberrant pericytes and basement
membrane coverage, overexpression of specific surface
receptor or antigen, and the presence of negative charged
macro-molecules for example, anionic phospholipids
and glycoprotein. Based on these characters, several
strategies have been used to develop neo-vascular targeting
liposomal drugs, which include conjugating with specific
antibody again surface antigen or receptor and modified,
non-functional receptor binding ligand, or incorporating
positive (cationic) charged molecules in the surface of
liposome. Of them, cationic liposome is a unique and
interesting approach ( 31). In a preclinical study, Kalra and
Campbell showed 5-FU and doxorubicin-loaded cationic
liposome could preferentially bind with human endothelial
(HMEC-1 and HUVEC) rather than pancreatic cancer
cells. (HPAF-II and Capan-1)( 32). Subsequently, Eichhorn
et al showed that both cationic lipid complexed paclitaxel
(EndoTAG TM-1) and camptothecin (EndoTAG TM-2) could
preferentially bind at endothelial cells of neo-vasculature
in solid tumor preclinical model ( 33-35). The selectively
targeting of both agents on tumor microvasculature was
confirmed by quantitative fluorescence microscopy.
Further study suggested the anti-vascular effect of cationic
liposome encapsulated paclitaxel (EndoTAG TM-1) is
schedule-dependent with metronomic schedule better than the maximum tolerated dose schedule. In addition,
the combination of EndoTAG TM-1 and gemcitabine could
significantly inhibit the incidence of metastatsis in L3.6pl
orthotopic pancreatic cancer mice model. Based on these data, EndoTAG TM-1, a cationic liposome
(prepared from 1,2 dioleoyl-3-trimethyl- ammoniumpropane
(DOTAP) and 1,2 dioleoyl-sn-glycero-3-
phosphocholine (DOPC)) encapsulated paclitaxel, has
been used in combination with gemcitabine to treat chemonaïve
pancreatic cancer patients. The latest follow-up data of
the four-arm randomized, phase II trial comparing weekly
gemcitabine 1,000 mg/m 2 alone versus gemcitabine plus
twice weekly EndoTAG TM-1 at three different doses, 11, 22
and 44 mg/m 2) was presented in the 2009 ASCO Annual
Meeting ( 36). Of the 200 chemo-naïve advanced pancreatic
cancer patients who participated the study, 80% had
metastatic diseases and 20% had locally advanced diseases.
Disease-control rates in the gemcitabine monotherapy
arm and the three gemcitabine plus EndoTAG-1 arms
was 43% and ranging from 53% to 69%, respectively. The
median progression-free survival time in corresponding
group of patients were 2.7 months versus 4.1 to 4.6 months,
respectively. The median overall survival time of patients
receiving gemcitabine plus either high-dose (44 mg/m 2)
or intermediate-dose of EndoTAG-1 were 9.4 months and
8.7 months, respectively, as compared with the 7.2 months
in the gemcitabine monotherapy arm. The adjusted hazard
ratio for overall survival for either arm was 0.72 (95% CI,
0.46 to 1.13) and 0.67 (95% CI, 0.43 to 1.07), respectively.
The data is exciting but large-scale study to validate the data
is mandatory.
|
|
Polymeric Micelles
Polymeric micelles-based anticancer drug, consisting of the
incorporation of chemotherapeutic agent into polymeric
micelles in size of 20–100 nm, was originally developed
by Professor Kataoka( 37). The polymeric micelle has two
major components, a polyethylene glycol (PEG) constituted
hydrophilic outer shell and a cytotoxic chemotherapeutic
agent incorporated hydrophobic inner core. The main
action mechanism of the polymeric micelles is similar to
lipomosal agents and through the passive targeting based
on the enhanced permeability of tumor neo-vasculature
and the impeding clearance of macromolecules from
lymphatic-deficient tumor interstitial tissue. Several
cytotoxic chemotherapy-incorporating polymeric micellar
nanoparticles have been in clinical trials, including
paclitaxel-incorporating PEG-polyaspartate (NK105),
cisplatin-incorporating PEG-polyglutamate/cisplatin
complex (NC-6004) and SN-38-incorporating PEG-ployglutamate/SN-38 (NK012). Of them, NC-6004 is
currently evaluated in a phase Ib/II trial for patients with
advanced pancreatic cancer, and will be discussed ( 38-41). |
|
Cisplatin-incorporating Polymeric Micelles,
NC-6004
In animal study, NC-6004 showed characteristic delayed
total body clearance and higher area-under curve as
compared with free cisplatin with a ratio of 1/19 and 65
folds, respectively ( 42). In addition, both histopathological
and biochemical studies suggested NC-6004 significantly
reduced cisplatin-associated nephrotoxicity. In phase I trial
for patients with refractory advanced solid tumor, escalating
dose of NC-6004 was administered intravenously every
3 weeks. Despite the implantation of pre-medication
and post-therapy hydration, nephrotoxicity and allergic
reaction were observed in patients receiving 120 mg/m 2
and further dose escalation was withheld. The MTD and
the recommended dose were determined as 120 mg/m 2 and
90 mg/m 2, respectively. Pharmacokinetic study showed
the maximum plasma concentration and area under curve
of ultra-filterable platinum after 120 mg/m 2 of NC-6004
were 1/34 and 8.5 folds of those with free cisplatin ( 43).
Seven out of 17 accruals achieved stable diseases, including
two of two pancreatic cancer patients who had NC-6004
at dose level of 90 mg/m 2. Perhaps owing to earlier metaanalysis
showed he combination of gemcitabine and
platinum could significantly improved the overall survival
of advanced pancreatic cancer patients as compared to
gemcitabine monotherapy, NC-6004 is currently proceeded
into a phase Ib/II trial to evaluate the maximum tolerated
dose of NC-6004 in combination with gemcitabine and
the therapeutic efficacy of the combination in patients with
chemo-naïve advanced pancreatic cancer, clinicaltrials.gov
identifier NCT00910741. |
|
Rexin-G
Rexin-G is a highly engineered, nonreplicating retroviral
vector displaying a von Willebrand factor–derived collagenbinding
motif at its amphotropic envelope, and expressing a
dominant negative cyclin G1 gene ( 44-46). This Willebrand
factor-derived collagen-binding motif on the retrovector’
s surface enables the nanoparticle drug to seek and be
selectively delivered to primary and secondary tumor
sites where angiogenesis and collagen matrix exposure
characteristically occur. The encoded dominant negative
cyclin G1 gene will thus to disrupt tumor cell cyclin G1
activity to lead to the destruction and/or growth inhibition
of tumor. There were two dose escalating phase I trials evaluating
different dose/schedule of Rex in-G in patients with
gemcitabine-failed advanced pancreatic cancer. The first
trial evaluating 3 dose levels of Rexin-G administered
intravenously, level I, 7.5 x 10 9 colony forming units
(CFU) per day, days 1-7 and 15-21 every 28 days; level II,
1.1 x 10 10 CFU per day, days 1-7 and 15-21 every 28 days;
and level III, 3 x 10 10 CFU per day, 5 days per week x 4
weeks/cycle with 6 weeks rest between two cycles. A total
of 12 patients were enrolled, only one patient with doselimiting
toxicity manifesting as grade 3 transaminitis was
observed at dose level II. However, the best tumor response
was stable disease in one (8.3%) and the median time to
tumor progression and overall survival of intent-to-treat
population were 32 days and 3.5 months, respectively ( 47).
In the second trial, the dose of Rexin-G was increased to
1 x 10 11 CFU per day, twice or thrice per week for 4 weeks
as one cycle (dose levels 0 and I), and 2 x 10 11 CFU per day,
thrice per week for 4 weeks as one cycle (dose levels II). A
total of 13 patients were enrolled, 6 in dose level 0-I and 7
in dose level II. There was no DLT observed. On intent-to
–treat analysis, the tumor control rate was 50% (3/6) and
85.7% (6/7 with one partial responder) of patients at dose
level 0-I and II, respectively. The median overall survival
in corresponding group of patients was 2.6 months and 9.3
months, respectively ( 48). Based on the results, the US FDA
has granted Rexin-G fast-track designation as second-line
treatment for pancreatic cancer in June 2009. Currently, a
phase II/III pivotal two-arm randomized study aiming to
validate the survival benefit of Rexin-G monotherapy versus
physician’s choice in gemcitabine-refractory pancreatic
cancer is under discussion.
|
|
Conclusion
Systemic therapy for advanced pancreatic cancer has
been largely disappointed owing to the unfavorable
pharmacokinetic profile and poor penetration of current
chemotherapeutic agents and the fragile patient population
hard to tolerate toxic combinat ion chemotherapy.
Nanovector can provide passive or active targeting drug
delivery to reduce the system exposure and enhance
local drug retention in tumor tissue. In this review, we
provide pre-clinical and clinical evidence to support the
potential use of nanovector-based therapy in patients with
advanced pancreatic cancer. Unfortunately, most of trials
reported here are relatively small and without control
group. Prospective, large-scale randomization trials are
warranted to confirm their efficacy in this difficult tumor.
In addition, the combination of the relatively low toxic
nanoparticle drug with conventional cytotoxic agent and/or recently emergent molecular targeted agent should also be
investigated to improve the clinical outcomes of patients
with advanced pancreatic cancer.

|
|
References
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J
Clin 2010;60:277-300.[LinkOut]
- Cleary SP, Gryfe R, Guindi M, Greig P, Smith L, Mackenzie R, et al.
Prognostic factors in resected pancreatic adenocarcinoma: analysis of
actual 5-year survivors. J Am Coll Surg 2004 ;198:722-31.[LinkOut]
- Society AS: Cancer Facts & Figures 2010. Atlanta: American Cancer
Society; . 2010.
- Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, et al.
Adjuvant chemotherapy with gemcitabine vs observation in patients
undergoing curative-intent resection of pancreatic cancer: a randomized
controlled trial. JAMA 2007;297:267-77.[LinkOut]
- Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, et al.
Erlotinib plus gemcitabine compared with gemcitabine alone in patients
with advanced pancreatic cancer: a phase III trial of the National
Cancer Institute of Canada Clinical Trials Group. J Clin Oncol
2007;25:1960-6.[LinkOut]
- Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn
Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic
cancer. N Engl J Med 2011;364:1817-25.[LinkOut]
- Kim R. FOLFIRINOX: a new standard treatment for advanced
pancreatic cancer? Lancet Oncol 2011;12:8-9.[LinkOut]
- Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R.
Nanocar r iers as an emerging platform for cancer therapy. Nat
Nanotechnol 2007;2:751-60.[LinkOut]
- Schnitzer JE. gp60 is an albumin-binding glycoprotein expressed by
continuous endothelium involved in albumin transcytosis. Am J Physiol
1992;262:H246-54.[LinkOut]
- Gradishar WJ, Tjulandin S, Davidson N, Shaw H, Desai N, Bhar P, et al.
Phase III trial of nanoparticle albumin-bound paclitaxel compared with
polyethylated castor oil-based paclitaxel in women with breast cancer. J
Clin Oncol 2005;23:7794-803.[LinkOut]
- Schnitzer JE, Oh P. Antibodies to SPARC inhibit albumin binding to SPARC, gp60, and microvascular endothelium. Am J Physiol 1992;263:
H1872-9.[LinkOut]
- Ibrahim NK, Desai N, Legha S, Soon-Shiong P, Theriault RL, Rivera E,
et al. Phase I and pharmacokinetic study of ABI-007, a Cremophor-free,
protein-stabilized, nanoparticle formulation of paclitaxel. Clin Cancer
Res 2002;8:1038-44.[LinkOut]
- Von Hoff D, Board M, Ramanathan R, Smith L, Drengler R, Wood T,
et al. Promising clinical activity of a NAB paclitaxel plus gemcitabine
combination in a disease-specific phase I trial in patients with advanced
pancreatic cancer[abstract]. AACR Annual Meeting 2008; 4179.
- Hoff DDV, Ramanathan R, Borad M, Laheru D, Smith L, Wood T, et
al. SPARC correlation with response to gemcitabine (G) plus nabpaclitaxel
(nab-P) in patients with advanced metastatic pancreatic
cancer: A phase I/II study[abstract]. J Clin Oncol 2009;27:4525.
- El-Khoueiry AB, Iqbal S, Lenz H, Gitlitz BJ, Yang D, Cole S,et al. A
phase I study of two different schedules of nab-paclitaxel (nab-P) with
ascending doses of vandetanib (V) with expansion in patients (Pts) with
pancreatic cancer (PC)[abstract]. J Clin Oncol 2011;29:4124.
- Presant CA, Scolaro M, Kennedy P, Blayney DW, Flanagan B, Lisak J,
et al. Liposomal daunorubicin treatment of HIV-associated Kaposi’s
sarcoma. Lancet 1993;341:1242-3.[LinkOut]
- Masood R, Husain SR, Rahman A, Gill P. Potentiation of cytotoxicity
of Kaposi’s sarcoma related to immunodeficiency syndrome (AIDS)
by liposome-encapsulated doxorubicin. AIDS Res Hum Retroviruses
1993;9:741-6.[LinkOut]
- Tulpule A, Yung RC, Wernz J, Espina BM, Myers A, Scadden DT, et
al. Phase II trial of liposomal daunorubicin in the treatment of AIDSrelated
pulmonary Kaposi’s sarcoma. J Clin Oncol 1998;16:3369-74.[LinkOut]
- Vaage J, Donovan D, Uster P, Working P. Tumour uptake of doxorubicin
in polyethylene glycol-coated liposomes and therapeutic effect against a
xenografted human pancreatic carcinoma. Br J Cancer 1997;75:482-6.[LinkOut]
- Halford S, Yip D, Karapetis CS, Strickland AH, Steger A, Khawaja
HT, et al. A phase II study evaluating the tolerability and efficacy
of CAELYX (liposomal doxorubicin, Doxil) in the treatment of
unresectable pancreatic carcinoma. Ann Oncol 2001;12:1399-402.[LinkOut]
- Hofheinz RD, Willer A, Weisser A, Gnad U, Saussele S, Kreil S, et al.
Pegylated liposomal doxorubicin in combination with mitomycin
C, infusional 5-f luorouracil and sodium folinic acid. A phase-I-study in patients with upper gastrointestinal cancer. Br J Cancer
2004;90:1893-7.[LinkOut]
- Heinemann V, Labianca R, Hinke A, Louvet C. Increased survival using
platinum analog combined with gemcitabine as compared to singleagent
gemcitabine in advanced pancreatic cancer: pooled analysis of
two randomized trials, the GERCOR/GISCAD intergroup study and a
German multicenter study. Annals of oncology 2007;18:1652-9.[LinkOut]
- Boulikas T. Molecular mechanisms of cisplatin and its liposomally
encapsulated form, Lipoplatin™. Lipoplatin™ as a chemotherapy and
antiangiogenesis drug. Cancer Therapy 2007;5:351-76.
- Stathopoulos GP, Boulikas T, Vougiouka M, Deliconstantinos G,
Rigatos S, Darli E, et al. Pharmacokinetics and adverse reactions
of a new liposomal cisplatin (Lipoplatin): phase I study. Oncol Rep
2005;13:589-95.[LinkOut]
- Stathopoulos GP, Boulikas T, Vougiouka M, Rigatos SK, Stathopoulos
JG. Liposomal cisplatin combined with gemcitabine in pretreated
advanced pancreatic cancer patients: a phase I-II study. Oncol Rep
2006;15:1201-4.[LinkOut]
- Perez-Soler R, Lopez-Berestein G, Lautersztain J, al-Baker S, Francis
K, Macias-Kiger D, et al. Phase I clinical and pharmacological
study of liposome-entrapped cis-bis-neodecanoato-trans-R,R-1,2-
diaminocyclohexane platinum(II). Cancer Res 1990;50:4254-9.[LinkOut]
- Drummond DC, Noble CO, Guo Z, Hong K, Park JW, Kirpotin DB.
Development of a highly active nanoliposomal irinotecan using a novel
intraliposomal stabilization strategy. Cancer Res 2006;66:3271-7.[LinkOut]
- Hann B, Peth K, Wang D, Gysin S, Li S, Kullberg E, et al. Lipidic
nanoparticle CPT-11 in a bioluminescent orthotopic pancreas cancer
model[abstract]. AACR Meeting Abstracts 2007;5648.
- Chen L, Chang T, Cheng A, Yang C, Shiah H, Chang J, et al. Phase I
study of liposome encapsulated irinotecan (PEP02) in advanced solid
tumor patients[abstract]. J Clin Oncol 2008;26:2565.
- Ko AH, Tempero MA, Shan Y, Su W, Lin Y, Dito E, et al. A multinational
phase II study of liposome irinotecan (PEP02) for patients with
gemcitabine-refractory metastatic pancreatic cancer. J Clin Oncol
2011;29:s4.
- Schmitt-Sody M, Strieth S, Krasnici S, Sauer B, Schulze B, Teifel
M, et al. Neovascular targeting therapy: paclitaxel encapsulated in
cationic liposomes improves antitumoral efficacy. Clin Cancer Res
2003;9:2335-41.[LinkOut]
- Kalra AV, Campbell RB. Development of 5-FU and doxorubicin-loaded
cationic liposomes against human pancreatic cancer: Implications for
tumor vascular targeting. Pharm Res 2006;23:2809-17.[LinkOut]
- Eichhorn ME, Ischenko I, Luedemann S, Strieth S, Papyan A, Werner A,
et al. Vascular targeting by EndoTAG-1 enhances therapeutic efficacy of
conventional chemotherapy in lung and pancreatic cancer. Int J Cancer
2010;126:1235-45.[LinkOut]
- Eichhorn ME, Luedemann S, Strieth S, Papyan A, Ruhstorfer H,
Haas H, et al. Cationic lipid complexed camptothecin (EndoTAG-2)
improves antitumoral efficacy by tumor vascular targeting. Cancer Biol
Ther 2007;6:920-9.[LinkOut]
- Strieth S, Eichhorn ME, Sauer B, Schulze B, Teifel M, Michaelis U, et
al. Neovascular targeting chemotherapy: encapsulation of paclitaxel in
cationic liposomes impairs functional tumor microvasculature. Int J
Cancer 2004;110:117-24.[LinkOut]
- Loehr M, Bodoky G, Fölsch U, Märten A, Karrasch M, Lilla C, et al.
Cationic liposomal paclitaxel in combination with gemcitabine in
patients with advanced pancreatic cancer: A phase II trial. JCO suppl
2009;abstr 4526.
- Yokoyama M, Okano T, Sakurai Y, Suwa S, Kataoka k. Introduction
of cisplatin into polymeric micelle. Journal of Controlled Release
1996;39:351-356.[LinkOut]
- Hamaguchi T, Kato K, Yasui H, Morizane C, Ikeda M, Ueno H, et al. A
phase I and pharmacokinetic study of NK105, a paclitaxel-incorporating
micellar nanoparticle formulation. Br J Cancer 2007;97:170-6.[LinkOut]
- Matsumura Y. Preclinical and clinical studies of NK012, an SN-38-
incorporating polymeric micelles, which is designed based on EPR
effect. Adv Drug Deliv Rev 2011;63:184-92.[LinkOut]
- Saito Y, Yasunaga M, Kuroda J, Koga Y, Matsumura Y. Enhanced
distribution of NK012, a polymeric micelle-encapsulated SN-38, and
sustained release of SN-38 within tumors can beat a hypovascular
tumor. Cancer Sci 2008;99:1258-64.[LinkOut]
- Saito Y, Yasunaga M, Kuroda J, Koga Y, Matsumura Y. Antitumour
activity of NK012, SN-38-incorporating polymeric micelles,
in hypovascular orthotopic pancreatic tumour. Eur J Cancer
2010;46:650-8.[LinkOut]
- Uchino H, Matsumura Y, Negishi T, Koizumi F, Hayashi T, Honda
T, et al. Cisplatin-incorporating polymeric micelles (NC-6004) can
reduce nephrotoxicity and neurotoxicity of cisplatin in rats. Br J Cancer
2005;93:678-87.[LinkOut]
- Plummer R, Wilson RH, Calvert H, Boddy AV, Griffin M, Sludden J, et
al. A Phase I clinical study of cisplatin-incorporated polymeric micelles
(NC-6004) in patients with solid tumours. Br J Cancer 2011;104:593-8.[LinkOut]
- Gordon EM, Hall FL. The 'timely' development of Rexin-G: first
targeted injectable gene vector (review). Int J Oncol 2009;35:229-38.[LinkOut]
- Gordon EM, Hall FL. Rexin-G, a targeted genetic medicine for cancer.
Expert Opin Biol Ther 2010;10:819-32.[LinkOut]
- Gordon EM, Hall FL. Noteworthy clinical case studies in cancer gene
therapy: tumor-targeted Rexin-G advances as an efficacious anti-cancer
agent. Int J Oncol 2010;36:1341-53.[LinkOut]
- Galanis E, Carlson SK, Foster NR, Lowe V, Quevedo F, McWilliams
RR, et al. Phase I trial of a pathotropic retroviral vector expressing a
cytocidal cyclin G1 construct (Rexin-G) in patients with advanced
pancreatic cancer. Mol Ther 2008;16:979-84.[LinkOut]
- Chawla SP, Chua VS, Fernandez L, Quon D, Blackwelder WC, Gordon
EM, et al. Advanced phase I/II studies of targeted gene delivery in vivo:
intravenous Rexin-G for gemcitabine-resistant metastatic pancreatic
cancer. Mol Ther 2010;18:435-41.[LinkOut]
Cite this article as:
Tsai C, Park J, Chen L. Nanovector-based therapies in advanced pancreatic cancer. J Gastrointest Oncol. 2011;2(3):185-194. DOI:10.3978/j.issn.2078-6891.2011.034
|