Is there an optimal staging system or liver reserve model that can predict outcome in hepatocellular carcinoma?
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the second leading cause of cancer death worldwide (1). In the United States, the mortality has been rising in the past decades along with an uptrend in the incidence (2,3). Despite the improvement in HCC diagnosis and treatment, the survival is still poor with the median overall survival (OS) of 6 months (4). It is projected that the HCC incidence will continue to rise over the next decade (3). Understanding a patient’s prognosis is crucial in developing a treatment plan, especially since therapies like liver transplant are expensive and involve use of limited resources. Many staging systems and liver reserve models have been proposed to predict the HCC prognosis.
With regards to staging systems, none of the proposed staging systems has been universally accepted. Several studies comparing the predictive power of different staging systems have shown conflicting results. However, several studies have suggested that the two systems that are the best predictors include the Cancer of the Liver Italian Program (CLIP) staging system and the Barcelona Clinic Liver Cancer (BCLC) staging system (5-10). The CLIP incorporates tumor morphology and liver function (11). It has been suggested as the primary staging system since it is simple to use and has been well validated (12). The BCLC staging system also takes patient’s performance status into account, which is also be an important prognostic factor (13,14). To date, it is still unclear as to which system provides the best prognostication.
The majority of HCC patients have coexisting liver cirrhosis and liver functional reserve is one of the key prognostic factors. The Child-Pugh score (CP) was originally designed for predicting the outcome after surgery for portal hypertension in cirrhotic patients (15,16). This score appeared to be a robust predictor of survival and has been the reference for assessing the prognosis of cirrhosis in HCC patients (17). However, there are some limitations as this score consists of subjective variables (ascites and encephalopathy) and was designed for cirrhotic patients. In fact, many HCCs arise with no underlying cirrhosis (18). The Model for End-Stage Liver Disease (MELD) has been primarily used for allocation of allograft for liver transplantation. The MELD score has also shown to be a good mortality predictor in other populations including HCC (19). Other liver reserve markers including the FIB-4 score and aspartate aminotransferase-to-platelet ratio Index (APRI) have been proposed to assess liver dysfunction (20,21). To overcome the limitations of the CP score, the albumin-bilirubin (ALBI) grade was proposed as a simple and objective method for assessment of liver function in HCC (22) and has been shown to be superior to the CP score (23-25). The platelet-albumin-bilirubin (PALBI) grade was later developed by adding platelet count as a surrogate marker for portal hypertension (26). The PALBI grade had a superior prognostic power than the ALBI grade (27).
Although these staging systems and liver reserve models were not developed to predict HCC recurrence, the few studies, which have investigated their prognostic values in predicting HCC recurrence showed inconsistent results (28-32). Our study aimed to compare the ability of these 8 noninvasive models in predicting OS and disease-free survival (DFS) in a cohort of patients with HCC.
Methods
Patients and follow-up
Patients with newly diagnosed HCC who were referred to our group of physicians associated with the only liver transplant program in Hawaii, and the only referral center for liver disease for American territories of the Pacific Basin (including Samoa, Guam, Saipan, and the Marshall Islands) were prospectively collected from January 1, 1991 to June 30, 2017. Follow-up was censored on December 31, 2017. Patients who underwent liver transplantation were excluded since liver transplantation can improve survival by removing not only the tumor but also underlying cirrhosis. This study was approved by the Institutional Review Board of University of Hawaii.
All patients who underwent curative therapy were followed up with a computed tomography (CT) scan, as well as serological tests including liver function and alpha-fetoprotein (AFP) every 3 months for the first year then every 4–6 months in subsequent years with either a CT scan or an ultrasonography (USG). Any suspicious lesions of 1 cm of greater seen on an USG had a CT or a magnetic resonance imaging (MRI). If there was any suggestion of recurrence, the patient was presented to the multi-disciplinary hepatobiliary tumor conference that included hepatologists, oncologists, surgeons, pathologists, and radiologists. Biopsy was done in cases of doubt for recurrence. Additional therapy was based on recommendations from the committee. The date of recurrence, death, and last follow-up were recorded.
The primary clinical endpoint was OS, calculated from the date of diagnosis to the date of death or last follow-up. The secondary endpoint was DFS was defined from the date of treatment to the date of recurrence or death. Only patients who underwent treatment with curative intent were included in the analysis of DFS and recurrence. Curative treatments for this particular study included only surgical resection or locally ablative therapy. Survival status of all patients was obtained from hospital records as well as the Social Security Death Index and local newspaper (Star Advertiser) obituaries.
Diagnosis and treatment of HCC
HCC was diagnosed by either a histological or clinical diagnosis. Histological diagnosis of HCC was made either from a liver biopsy or an examination of the resected liver. Patients met a clinical diagnosis if they had a history of chronic liver disease, mass >2 cm in size on dynamic imaging and one of the following: (I) arterial uptake with venous washout seen on a CT scan or a MRI; (II) serum AFP >200 ng/mL.
Type of treatments included resection, ablative therapy (radiofrequency ablation, microwave ablation, cryotherapy and percutaneous ethanol injection), loco-regional therapy (Yttrium-90 radioembolization, transarterial chemoembolization), systemic therapy, and best supportive care. Patients were presented to a multidisciplinary HCC board for treatment discussion. Information of therapeutic risks and benefits was comprehensively provided to individual patient. Share decisions were made between patients and clinicians after counseling.
Data abstraction and variables
Demographic variables (age at diagnosis, sex), alcohol use, underlying diseases (diabetes, hypertension), viral hepatitis status (hepatitis B, hepatitis C), cirrhosis, tumor characteristics (maximal tumor diameter, number of tumors), and serum biochemistry were obtained from clinical interview and chart review. All data were determined at the time of HCC diagnosis and before therapy. We aimed to determine which noninvasive models were the best in predicting OS and DFS in our HCC cohort. This included the following models: 2 staging systems (CLIP and BCLC) and 6 liver reserve models (ALBI, APRI, CP, FIB-4, MELD, and PALBI). These noninvasive models were calculated according to their original formulas, and grading of severity was classified at the time of diagnosis according to the scores. The ALBI grade is classified into 3 grades (≤−2.60/>−2.60 and ≤−1.39/>−1.39) (22). The APRI score is classified into 3 grades (<0.5/0.5–1.5/>1.5) (20). The FIB-4 score is classified into 3 grades (<1.45/1.45–3.25/>3.25) (21). The MELD score is stratified into three risk groups (<10/10–14/>14) as previously proposed (33). The PALBI grade is classified into 3 grades (≤−2.53/>−2.53 and ≤−2.09/> 2.09) (26).
Statistical analysis
The survival distributions for the noninvasive models were examined by the Kaplan-Meier methods and compared by the log-rank test. The Cox regression models were fitted to derive hazard ratios (HR) of the effect of each noninvasive model on OS and DFS after adjusting for other factors in a multivariable model. In the multivariate analyses, all models were adjusted for age, sex, hepatitis B, hepatitis C, alcohol use, diabetes, AFP, number of tumors, maximal tumor diameter. To evaluate the discriminatory abilities for predicting mortality and recurrence at 1- and 3-year intervals, the area under the receiver operator characteristic curve (AUC) was calculated and compared for each noninvasive model. When calculating the AUC, the simplified grades were tested. A subgroup analysis was performed according to treatment strategies (curative treatment and non-curative treatment). P value <0.05 was considered statistically significant. All statistical analyses were conducted using IBM SPSS Statistics (version 21.0. Armonk, NY, USA).
Results
Baseline patient characteristics
During the study period, a total of 900 newly diagnosed HCC patients were identified. The majority of the patients were male (73.3%) with a mean age of 63 years. The patients were Asians (60.1%), Whites (18.8%), Hawaiians and Pacific Islanders (16.1%), mixed races (2.3%), Hispanics (1.9%), and Blacks (0.8%), respectively. History of alcohol use, hepatitis B infection, hepatitis C infection, and cirrhosis were accounted for 41.2%, 25.9%, 40.6%, and 66.6% of all patients, respectively. The majority of the patients were in CP class A (69.3%). Surgical resection, ablation therapy, loco-regional therapy, systemic therapy, and best supportive care were performed in 22.2%, 25.2%, 24.8%, 9.2%, and 18.6% of the patients, respectively. Table 1 describes the patient, tumor, and treatment characteristics of all patients. The median follow-up duration was 19.8 months and the mean follow-up duration was 33.2 months.
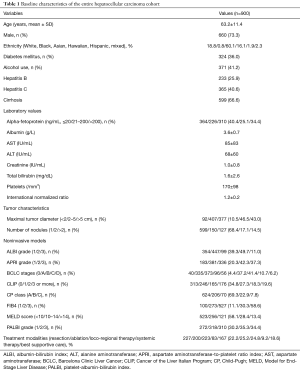
Full table
HCC mortality
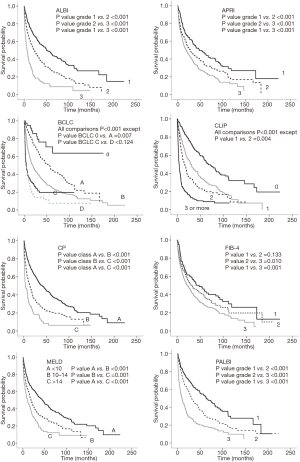
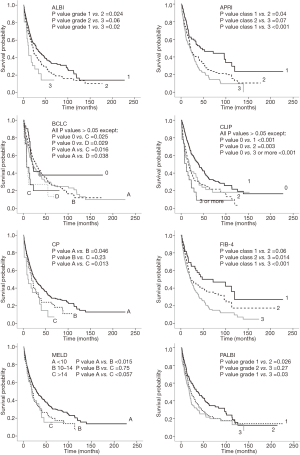
There were 598 (66.4%) deaths with a median survival time of 27.4 months (95% CI, 23.0–32.0 months). In a multivariable Cox proportional hazards model, all noninvasive models were independently associated with OS and each model showed a significant difference in the probability of survival across the different stages (Table 2). The Kaplan-Meier distributions of OS according to each noninvasive model are shown in Figure 1. Significant differences in survival distribution were found across all strata of all models with an exception of the FIB-4 score and the BCLC stage.
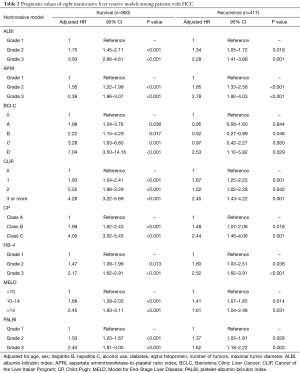
Full table
The discriminatory abilities of 8 models for mortality were tested by the AUC method (Table 3). At 1- and 3-year intervals, the CLIP score provided the highest AUC value, followed by the BCLC stage and the PALBI grade, respectively. The FIB-4 score had the lowest AUC value at both 1- and 3-year intervals.
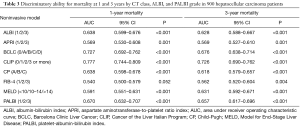
Full table
A subgroup analysis according to treatment strategies demonstrated the same findings as the main analysis. The included staging systems had a higher prognostic power than the included liver reserve models. The CLIP score had a higher prognostic power than the BCLC staging system in both groups (patients undergoing curative treatment and non-curative treatment). The PALBI had the highest prognostic power of all liver reserve models in both groups (Table S1).
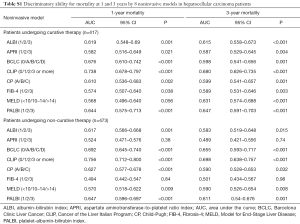
Full table
HCC recurrence
A total of 427 patients underwent curative treatment. Ten patients were excluded due to not having adequate data. Four hundred seventeen HCC patients were included in the analysis. There were 288 patients (69.1%) who had HCC recurrence or died with a median time of 23.1 months (95% CI, 18.7–27.5 months). In a multivariable Cox proportional hazards model, all noninvasive models were independently associated with DFS and each model showed a significant difference in the probability of survival across the different stages except for the BCLC stage (Table 2). The Kaplan-Meier distributions of DFS according to each model are shown in Figure 2. None of the included models showed significant differences in survival distribution across all strata. For predicting 1-year recurrence, the BCLC system had the highest discriminative power followed by the PALBI grade, ALBI grade, and CLIP score. For predicting 3-year recurrence, the CLIP score demonstrated the highest discriminative power followed by the PALBI grade, ALBI grade and BCLC system, respectively. However, they overall were found to be fair predictors for recurrence (Table 4).
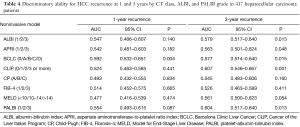
Full table
Discussion
There has been much debate as to which of the staging systems and liver reserve models have the best prognostic power for HCC. Our study used data from a large prospective cohort of HCC patients from early to advanced cancer stage undergoing different treatment modalities. In this study, the included staging systems demonstrated higher prognostic powers than the included liver reserve models. Regarding the staging systems, the CLIP score could predict both OS and DFS more accurately than the BCLC staging system. Regarding the liver reserve models, PALBI appeared to be the best model to predict both OS and DFS compared to the other models.
The staging systems were found to convey more prognostic information than the liver reserve models likely because they take into account key prognostic factors besides liver function including tumor characteristics and patient’s performance status (5). Our study assessed two frequently used staging systems (the CLIP score and the BCLC staging system). Using the Kaplan-Meier analysis, we found that both systems revealed a progressive decrease in OS from the earliest to most advanced stage. However, the CLIP score provided a higher prognostic power for OS than the BCLC system based on the AUC method and also in subgroups of patients undergoing curative treatment and non-curative treatment. The BCLC system seems to be the most comprehensive since it integrates key elements for prognostication including tumor characteristics, patient’s functional status and liver function. However, this model is not perfect as it was not designed to be a prognostic model like CLIP, which was based on multivariate analysis of a cohort of HCC patients. Another drawback is its rigidity. For example, any patients with a performance status equal to 1 (restricted in physical strenuous activity but able to carry out light work) fall into the advanced stage (BCLC C) regardless of tumor stage and liver function. The CLIP score was derived from 16 Italian institutions (11) and has been externally validated in other countries (10,34,35). Our findings are consistent with several previous studies (7,8,10,33,36). Furthermore, the CLIP score takes into account tumor characteristics, liver functional reserve and a biomarker (serum AFP) (11). Although serum AFP is not the best biomarker and several other biomarkers have been developed, none has been found so far to accomplish the clinical demand for optimal HCC patient care (37). With advances in cancer biology and molecular and genetic profiling, there are multiple proposed biologic explanations involved in the progression and prognosis of HCC, however there is no one unifying theory (38).
Liver functional reserve is one of the key prognostic factors however each scoring system has limitations and no system has evolved that can be universally applicable in the heterogenous HCC population. CP score is widely used to assess severity of liver cirrhosis in HCC patients. However, this score is limited by equal weighing of 5 parameters, its arbitrary cut-off values, 2 of them are subjective (ascites and encephalopathy), and was designed for cirrhotic patients. In fact, one-third of our patients had no underlying cirrhosis. The MELD score was initially developed to determine prognosis after portal hypertension procedure but has evolved the principal tool for prioritization and allocation in liver transplantation. FIB-4 and APRI were also developed to assess severity of liver cirrhosis. Their role for prognostication in non-cirrhotic HCC patients is limited. The ALBI and PALBI grades were recently developed to specifically assess hepatic dysfunction in HCC patients, which included only objective measures. A recent study showed that both ALBI and PALBI were able to predict survival more accurately than other liver reserve models including CP and MELD and the PALBI grade was superior to the ALBI grade (27). Our study supports this finding by demonstrating the superior prognostic power of PALBI over ALBI over the other models.
The included staging systems were not developed for use in predicting HCC recurrence after curative treatment. Only a few studies have investigated the role of these noninvasive models in predicting HCC recurrence and these showed inconsistent findings (30-32). Known risk factors for HCC recurrence include tumor characteristics, liver function and serum AFP, which are the composites of the staging systems (39-41). Our study found that both of them are fair predictors for HCC recurrence and one was not superior to the other. As liver function also one of the risk factors for HCC recurrence, a few studies have investigated the role of liver reserve models in predicting HCC recurrence (28,29). APRI was found to be a good predictor for HCC recurrence after RFA (28). Our study demonstrated that all 6 liver reserve models are found not to be good predictors for HCC recurrence.
Several limitations in this study should be acknowledged. First, this is a single-center study, in which patients are referred to a single group of surgeons and the results may not be generalizable. Second, being the only referral center for liver disease for American territories of the Pacific Basin, referral bias cannot be completely avoided. Third, treatment decisions were decided by the patients and the multi-disciplinary hepatobiliary tumor board based on shared decision making. Some patients might not strictly follow BCLC recommendations.
In conclusion, our results suggest that the staging systems demonstrated a higher predictive power than the liver reserve models. Regarding staging systems, the CLIP score is more accurate prognostic model to predict OS and DFS than the BCLC stage. The performance of CLIP score is reliable among different treatment strategies. Regarding the liver reserve models, the PALBI is the most accurate prognostic models among 6 models to predict OS and DFS. Further study is needed to address whether incorporating the PALBI grade instead of the conventional CP score into the CLIP staging system would increase the prognostic power to predict HCC survival that would further help guide treatment decisions.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Our study was approved by the University of Hawaii Institutional Review Board (protocol number: 2017-00517). Informed consent was obtained from each participant.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Beal EW, Tumin D, Kabir A, et al. Trends in the Mortality of Hepatocellular Carcinoma in the United States. J Gastrointest Surg 2017;21:2033-8. [Crossref] [PubMed]
- Petrick JL, Kelly SP, Altekruse SF, et al. Future of Hepatocellular Carcinoma Incidence in the United States Forecast Through 2030. J Clin Oncol 2016;34:1787-94. [Crossref] [PubMed]
- Njei B, Rotman Y, Ditah I, et al. Emerging trends in hepatocellular carcinoma incidence and mortality. Hepatology 2015;61:191-9. [Crossref] [PubMed]
- Marrero JA, Fontana RJ, Barrat A, et al. Prognosis of hepatocellular carcinoma: comparison of 7 staging systems in an American cohort. Hepatology 2005;41:707-16. [Crossref] [PubMed]
- Kim BK, Kim SU, Park JY, et al. Applicability of BCLC stage for prognostic stratification in comparison with other staging systems: single centre experience from long-term clinical outcomes of 1717 treatment-naive patients with hepatocellular carcinoma. Liver Int 2012;32:1120-7. [Crossref] [PubMed]
- Hsu CY, Hsia CY, Huang YH, et al. Selecting an optimal staging system for hepatocellular carcinoma: comparison of 5 currently used prognostic models. Cancer 2010;116:3006-14. [Crossref] [PubMed]
- Lin CY, Kee KM, Wang JH, et al. Is the Cancer of the Liver Italian Program system an adequate weighting for survival of hepatocellular carcinoma? Evaluation of intrascore prognostic value among 36 subgroups. Liver Int 2009;29:74-81. [Crossref] [PubMed]
- Cillo U, Bassanello M, Vitale A, et al. The critical issue of hepatocellular carcinoma prognostic classification: which is the best tool available? J Hepatol 2004;40:124-31. [Crossref] [PubMed]
- Levy I, Sherman M. Liver Cancer Study Group of the University of Toronto. Staging of hepatocellular carcinoma: assessment of the CLIP, Okuda, and Child-Pugh staging systems in a cohort of 257 patients in Toronto. Gut 2002;50:881-5. [Crossref] [PubMed]
- A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology 1998;28:751-5. [Crossref] [PubMed]
- Prospective validation of the CLIP score: a new prognostic system for patients with cirrhosis and hepatocellular carcinoma. The Cancer of the Liver Italian Program (CLIP) Investigators. Hepatology 2000;31:840-5. [Crossref] [PubMed]
- Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol 2001;35:421-30. [Crossref] [PubMed]
- Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 1999;19:329-38. [Crossref] [PubMed]
- Child CG, Turcotte JG. Surgery and portal hypertension. Major Probl Clin Surg 1964;1:1-85. [PubMed]
- Pugh RN, Murray-Lyon IM, Dawson JL, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973;60:646-9. [Crossref] [PubMed]
- D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol 2006;44:217-31. [Crossref] [PubMed]
- Gaddikeri S, McNeeley MF, Wang CL, et al. Hepatocellular carcinoma in the noncirrhotic liver. AJR Am J Roentgenol 2014;203. [Crossref] [PubMed]
- Kamath PS, Kim WR. Advanced Liver Disease Study G. The model for end-stage liver disease (MELD). Hepatology 2007;45:797-805. [Crossref] [PubMed]
- Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003;38:518-26. [Crossref] [PubMed]
- Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 2007;46:32-6. [Crossref] [PubMed]
- Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol 2015;33:550-8. [Crossref] [PubMed]
- Wang YY, Zhong JH, Su ZY, et al. Albumin-bilirubin versus Child-Pugh score as a predictor of outcome after liver resection for hepatocellular carcinoma. Br J Surg 2016;103:725-34. [Crossref] [PubMed]
- Toyoda H, Lai PB, O'Beirne J, et al. Long-term impact of liver function on curative therapy for hepatocellular carcinoma: application of the ALBI grade. Br J Cancer 2016;114:744-50. [Crossref] [PubMed]
- Hiraoka A, Kumada T, Michitaka K, et al. Usefulness of albumin-bilirubin grade for evaluation of prognosis of 2584 Japanese patients with hepatocellular carcinoma. J Gastroenterol Hepatol 2016;31:1031-6. [Crossref] [PubMed]
- Roayaie S, Jibara G, Berhane S, et al. editors. PALBI-An Objective Score Based on Platelets, Albumin & Bilirubin Stratifies HCC Patients Undergoing Resection & Ablation Better than Child's Classification. San Francisco: Association for the Study of Liver Diseases, 2015.
- Liu PH, Hsu CY, Hsia CY, et al. ALBI and PALBI grade predict survival for HCC across treatment modalities and BCLC stages in the MELD Era. J Gastroenterol Hepatol 2017;32:879-86. [Crossref] [PubMed]
- Chung HA, Kim JH, Hwang Y, et al. Noninvasive fibrosis marker can predict recurrence of hepatocellular carcinoma after radiofrequency ablation. Saudi J Gastroenterol 2016;22:57-63. [Crossref] [PubMed]
- Seo JY, Kim W, Kwon JH, et al. Noninvasive fibrosis indices predict intrahepatic distant recurrence of hepatitis B-related hepatocellular carcinoma following radiofrequency ablation. Liver Int 2013;33:884-93. [Crossref] [PubMed]
- Sirivatanauksorn Y, Tovikkai C. Comparison of staging systems of hepatocellular carcinoma. HPB Surg 2011;2011. [Crossref] [PubMed]
- Tokumitsu Y, Sakamoto K, Tokuhisa Y, et al. A new prognostic model for hepatocellular carcinoma recurrence after curative hepatectomy. Oncol Lett 2018;15:4411-22. [PubMed]
- Zhao WH, Ma ZM, Zhou XR, et al. Prediction of recurrence and prognosis in patients with hepatocellular carcinoma after resection by use of CLIP score. World J Gastroenterol 2002;8:237-42. [Crossref] [PubMed]
- Huo TI, Huang YH, Lin HC, et al. Proposal of a modified Cancer of the Liver Italian Program staging system based on the model for end-stage liver disease for patients with hepatocellular carcinoma undergoing loco-regional therapy. Am J Gastroenterol 2006;101:975-82. [Crossref] [PubMed]
- Farinati F, Rinaldi M, Gianni S, et al. How should patients with hepatocellular carcinoma be staged? Validation of a new prognostic system. Cancer 2000;89:2266-73. [Crossref] [PubMed]
- Ueno S, Tanabe G, Sako K, et al. Discrimination value of the new western prognostic system (CLIP score) for hepatocellular carcinoma in 662 Japanese patients. Cancer of the Liver Italian Program. Hepatology 2001;34:529-34. [Crossref] [PubMed]
- Camma C, Di Marco V, Cabibbo G, et al. Survival of patients with hepatocellular carcinoma in cirrhosis: a comparison of BCLC, CLIP and GRETCH staging systems. Aliment Pharmacol Ther 2008;28:62-75. [Crossref] [PubMed]
- Chaiteerakij R, Addissie BD, Roberts LR. Update on biomarkers of hepatocellular carcinoma. Clin Gastroenterol Hepatol 2015;13:237-45. [Crossref] [PubMed]
- Dhanasekaran R, Bandoh S, Roberts LR. Molecular pathogenesis of hepatocellular carcinoma and impact of therapeutic advances. F1000Res 2016.5. [PubMed]
- Park UJ, Kim YH, Kang KJ, et al. Risk factors for early recurrence after surgical resection for hepatocellular carcinoma. Korean J Hepatol 2008;14:371-80. [Crossref] [PubMed]
- Tateishi R, Shiina S, Yoshida H, et al. Prediction of recurrence of hepatocellular carcinoma after curative ablation using three tumor markers. Hepatology 2006;44:1518-27. [Crossref] [PubMed]
- Hung IF, Wong DK, Poon RT, et al. Risk Factors and Post-Resection Independent Predictive Score for the Recurrence of Hepatitis B-Related Hepatocellular Carcinoma. PLoS One 2016;11. [Crossref] [PubMed]


