Chemotherapy re-challenge response rate in metastatic colorectal cancer
Introduction
Colorectal cancer (CRC) is the second leading cause of cancer-related deaths in the United States and it is estimated that approximately 50,000 patients died from this disease in 2017 (1,2). Although CRC is the fourth most frequently diagnosed cancer, only 40% of patients are diagnosed during early stage disease (1,2). Treatment for stage II and stage III CRC consists of surgery followed by adjuvant chemotherapy with fluoropyrimidines (3). In high risk, early stage patients, the addition of oxaliplatin (FOLFOX) to standard therapy demonstrated an overall survival benefit as well as a 20% reduction in the risk of relapse in the MOSAIC trial (4). However, despite the use of aggressive therapy in stage II and stage III CRC, it is estimated that approximately 30% of these patients will develop metastatic disease (2-4).
The standard of care for metastatic colorectal cancer (mCRC) remains fluoropyrimidine-based therapy, often given with leucovorin and either oxaliplatin (FOLFOX) or irinotecan (FOLFIRI) (5-7). These regimens may also be administered in combination with monoclonal antibodies such as vascular endothelial growth factor (VEGF) inhibitors or epithelial growth factor receptor (EGFR) targeted therapies depending on RAS mutation status (8-11). Following frontline therapy in the metastatic setting, patients may elect to have chemotherapy holidays or receive ongoing maintenance therapy with fluoropyrimidines and a monoclonal antibody (12-14).
Once there is disease progression in mCRC, it is recommended to interchange cytotoxic drugs to include an agent the patient has not yet received (3). This practice is based on the widely-accepted concept that tumor cells can develop resistance to chemotherapy resulting in disease progression during or after a period of time from initial drug exposure (15,16). Due to acquired drug resistance, a different drug will be needed to elicit further response (15,16). Unfortunately, in mCRC the two main cytotoxic drugs utilized are oxaliplatin and irinotecan and after progression on both of these medications very few options remain (3).
Currently, the guideline-recommended third and fourth line therapies for refractory mCRC are regorafenib or trifluridine-tipiracil (3,17,18). While the development of these medications has resulted in additional treatment alternatives, the clinical benefit of these agents is limited, as progression free survival is approximately 1.9 months for regorafenib and 2.0 months for trifluridine-tipiracil (17,18). Furthermore, both regorafenib and trifluridine-tipiracil yielded minimal objective response rates, suggesting that these agents may prolong disease stability but are not optimal for cytotoxicity (17,18).
Another third or fourth line option for refractory mCRC may be the re-initiation of previous chemotherapy (19-23). While chemotherapy re-challenge has become a common practice in this patient population, there is limited data to support its efficacy (19-23). A recently published study assessed oxaliplatin-based retreatment in 83 patients (REOX) and found that 56.6% of study subjects obtained disease control, defined as a composite measure of complete response, partial response and stable disease (23). The REOX study also reported a median time to treatment failure of 6.04 months for oxaliplatin-based re-challenge, which compares favorably to regorafenib and trifluridine-tipiracil (17,18,23).
While existing data supporting oxaliplatin-based chemotherapy re-challenge in the third or fourth line setting for mCRC is promising, previous studies have not evaluated the efficacy of irinotecan-based re-challenge. The utilization of chemotherapy re-challenge in patients who have previously progressed during initial exposure has also remained in question. Therefore, further investigation into the optimal utilization and efficacy of chemotherapy re-challenge is required. In this retrospective study, the clinical benefit of an oxaliplatin or irinotecan-based chemotherapy re-challenge regimen was assessed in mCRC patients.
Methods
This was a retrospective, cohort study approved by the Institutional Review Board at a single outpatient cancer center. Pharmacy medication dispensing records at Banner MD Anderson Cancer Center from September 30, 2011–September 30, 2016 were utilized to identify patients who met inclusion criteria. Eligible patients had to be 18–89 years of age with known mCRC who received chemotherapy re-challenge. Chemotherapy re-challenge was defined as re-initiation of oxaliplatin or irinotecan-based regimens at least nine months from the end of initial exposure. A minimum of four chemotherapy cycles during the initial exposure was also required.
The key endpoints of this study were to determine clinical benefit rate (CBR), which was defined as the proportion of patients with partial response or stable disease, and time to progression (TTP). Response was determined through RECIST criteria and evaluation of previous radiology reports. TTP was defined as the time period from the initiation of re-challenge therapy to discontinuation due to progression of disease, toxicity, loss to follow-up, or death. Secondary endpoints included objective response rate, CBR associated with each re-challenge drug, and overall survival following chemotherapy re-challenge.
Statistical analysis
Descriptive statistics were used to quantify baseline demographic information and general characteristics. The primary end points were CBR and TTP associated with chemotherapy re-challenge. Impact on the primary endpoints by categorical variables such as re-challenge drug, gender, baseline performance status, RAS mutation status, and sites of metastases prior to re-challenge were assessed using the χ2 or Fisher exact test and logistic regression analysis. The Kruskall-Wallis test was used to compare the distributions of different independent variables on TTP. Correlation between duration of chemotherapy re-challenge and duration of response was determined by calculating the R2 or the Pearson correlation coefficient. Time of death from the end of re-challenge therapy was used to generate survival analysis using the Kaplan-Meier method.
Results
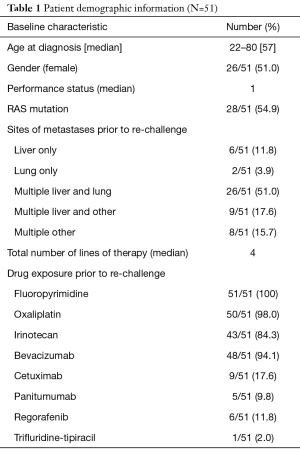
A total of 67 administrations of chemotherapy re-challenge were identified in 51 patients. Baseline demographics and distribution of exposure to different cytotoxic drugs prior to initiation of chemotherapy re-challenge are illustrated in Table 1. Overall, 51 patients received at least one re-challenge chemotherapy regimen and 14 of those 51 (27.4%) patients received multiple re-challenge regimens. The median age was 57 and median ECOG performance status was 1. The median total number of lines of therapy was 4, including chemotherapy re-challenge.
Full table
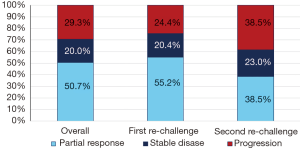
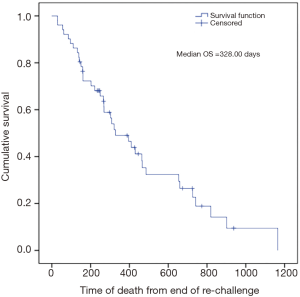
The overall CBR with chemotherapy re-challenge was 70.7% with 50.7% of patients experiencing a partial response and 20.0% having stable disease (Figure 1). No complete responses were observed. CBR was higher during the first re-challenge at 75.6% and then decreased during the second re-challenge to 61.5%. Similarly, the median TTP after the initiation of first chemotherapy re-challenge was 6.6 months, which decreased to 4.2 months after the second re-challenge. Overall TTP was 6.0 months (Table 2). Median time of death from the end of chemotherapy re-challenge was approximately 10.7 months (Figure 2).

Full table
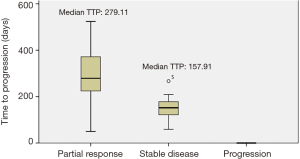
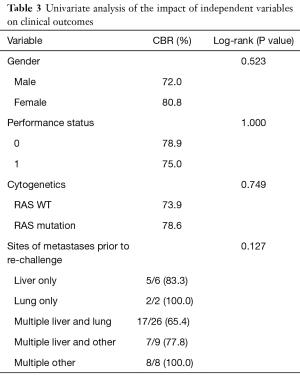
Independent variables such as gender, performance status, cytogenetics, and sites of metastases did not have a statistically significant impact on CBR (Table 3). The efficacy of each re-challenge drug was also assessed. During the first re-challenge, 66.7% of the chemotherapy regimens contained oxaliplatin and 33.3% irinotecan (Table 4). Median TTP with oxaliplatin was 171.50 days versus 240.00 days with irinotecan (P=0.258). CBR was higher with irinotecan at 88.2% versus 70.5% with oxaliplatin, but this difference was not statistically significant (P=0.317). TTP was also compared for patients who experienced a partial response during first re-challenge to those patients who obtained stable disease (Figure 3). Median TTP was 279.11 days in the partial response group and 157.91 days in the stable disease group (P=0.003).
Full table

Full table
There were 10 cases of chemotherapy re-challenge administered in patients who had previously progressed during the initial exposure (Table 5). The re-challenge partial response rate in these patients was 30.0%, and 40.0% of patients had stable disease. This CBR of 70.0% was similar to the overall CBR of 70.7%, however, the partial response rate was lower than the 50.7% overall partial response rate.
Reasons for discontinuation of chemotherapy re-challenge included disease progression (52.2%), chemotherapy holiday (8.9%), toxicity (23.9%), or transition to maintenance therapy (13.4%). There was also one patient who was receiving ongoing chemotherapy re-challenge at the time of data analysis.
Discussion
Our study evaluated the efficacy associated with chemotherapy re-challenge for refractory mCRC at a single, outpatient cancer center. Results from this study indicate that up to 70% of patients may experience clinical benefit from chemotherapy re-challenge as a third or fourth line option for mCRC despite prior exposure to irinotecan or oxaliplatin.
One of the first studies looking at the re-initiation of previously used chemotherapy in mCRC was the OPTIMOX1 trial published in 2006 (22). In this study, six hundred and twenty patients were randomized to receive FOLFOX every 2 weeks until progression or FOLFOX for 6 cycles, then maintenance therapy without oxaliplatin for 12 cycles, followed by reintroduction of FOLFOX (22). The main rationale for the design of this study was to assess whether a stop-and-go approach of oxaliplatin in mCRC could yield similar clinical outcomes as continuous therapy and minimize oxaliplatin induced neurotoxicity (22). Results from this trial determined that the difference in overall survival was not statistically significant between the two treatment arms nor was the rate of grade 3 sensory neuropathy (22). Furthermore, in the 89 patients in the OPTIMOX1 trial who were re-introduced to FOLFOX after progression, 49.4% of these patients experienced response or stabilization of disease, which was a lower CBR than the results of this study (22). Our study also demonstrated a longer TTP of 25 weeks on chemotherapy re-challenge compared to the OPTIMOX1 trial, which reported a median PFS with oxaliplatin of 12 weeks (22). These differences may be attributable to the continued use of monoclonal antibodies with concurrent cytotoxic therapy in our study population, only chemotherapy was administered in the OPTIMOX1 subjects (22).
Another study which assessed the efficacy of chemotherapy re-challenge was the RE-OPEN trial (23). In this phase II study, thirty-three patients who had progressed on prior chemotherapy with oxaliplatin and irinotecan were given oxaliplatin >6 months from the time of progression (23). Overall disease control rate was reported in 66.7% of patients following 12 weeks of oxaliplatin re-exposure (23). The median PFS was 98.0 days and median OS was 300.0 days (23). Interestingly, in the multivariate analysis for the RE-OPEN trial, factors that made a statistically significant impact on OS and PFS included ECOG performance status, number of metastatic sites, and gender (23). Specifically, patients who were male, had only 1 site of metastases, or an ECOG performance status of 0 were more likely to have favorable outcomes (23). Conversely, in the multivariate analysis for this study, there was no statistical significance detected with these demographic factors and influence on OS or TTP.
One unique aspect of the RE-OPEN trial was the inclusion of patients known to have confirmed progression on previous oxaliplatin-based therapy (23). The response rate of oxaliplatin re-challenge for these patients was reported at 6.1%, while 33.3% had stable disease and 54.5% progressed at 12 weeks (23). A majority of patients in our study did not have confirmed progression during initial exposure, and front-line therapy was typically stopped due to toxicity or to transition to a chemotherapy holiday or maintenance therapy. However, we were able to identify 10 cases of chemotherapy re-challenge in patients who had previously progressed on the same cytotoxic regimen. Thirty percent of patients achieved a partial response, while 40.0% of patients had stable disease and 30.0% of patients progressed. Although the partial response rate in this specific patient group was lower when compared to the 50.7% of overall responses in our study, the findings of the RE-OPEN trial and our study indicate that even patients with prior progression on chemotherapy may derive clinical benefit from re-challenge (23). The ability of previously failed chemotherapy to yield clinical benefit as a subsequent line of therapy suggests that colorectal tumor cells may undergo a series of genetic changes throughout treatment duration that alter drug sensitivity and resistance patterns. Mechanisms for these genetic changes still have yet to be well defined.
The efficacy of chemotherapy re-challenge was also assessed in the retrospective REOX trial (25). In this study, eighty-three patients were included if they had re-exposure to an oxaliplatin-containing regimen after previous failure (25). The median time to treatment failure after re-exposure was reported as 6.04 months, which was comparable to the TTP of 6.60 months associated with first chemotherapy re-challenge in our study and the overall TTP of 6.00 months (25). However, higher CBRs were associated with our study cohort versus the 56.6% of REOX patients who achieved disease control (25). Of note, similar to our study, there was no statistically significant impact associated between age, gender, cytogenetics, or performance status and favorable clinical outcomes (25).
When assessing other factors that may influence TTP and OS in chemotherapy re-challenge patients, one predictor identified in this study was the type of clinical benefit experienced. Specifically, patients who had a partial response to chemotherapy re-challenge were more likely to have a longer TTP than those patients with disease stabilization. This finding may be helpful in identifying individuals likely to gain the most benefit from chemotherapy re-challenge, especially in the setting of heavy pretreatment and concerns for cumulative toxicity. In the cohort evaluated in this study, 23.9% of re-challenge therapy was discontinued due to toxicity, indicating that a majority of patients were able to tolerate chemotherapy re-challenge but had to stop therapy due to progression (52.2%).
In comparison to the other aforementioned studies, a few unique aspects of our study included assessment of the efficacy of both oxaliplatin and irinotecan as re-challenge agents and analysis of patients who received multiple re-challenge regimens. While there was no statistically significant difference in the efficacy of oxaliplatin versus irinotecan re-challenge, the increase of median TTP with irinotecan may be suggestive of a possible added benefit with the use of irinotecan over oxaliplatin in the re-challenge setting. Persistent, chronic oxaliplatin-induced neurotoxicity may also be a concern when re-initiating therapy. For patients who received multiple re-challenge regimens, clinical benefit decreased with subsequent lines of treatment, however, because there were only fourteen cases of multiple re-challenge, further investigation with larger sample sizes is needed to draw conclusions on this outcome. Interestingly, the TTP associated with second re-challenge was 4.2 months, which compares favorably to the PFS associated with agents such as regorafenib and trifluridine-tipiracil. Ideal candidates for chemotherapy re-challenge may also be younger patients who have maintained a good performance status as the median ages in previously published studies was 53.5–62 and ECOG 0-1 (22,23,25).
Limitations of this study were primarily its retrospective nature and dependence on available documentation in the electronic medical record. Another limitation was the small number of patients who met inclusion criteria. Due to this small number, our findings may be hypothesis generating but cannot definitively determine the ideal utilization and role of chemotherapy re-challenge in this patient population. For the demographic and efficacy analysis, the completion of surgical procedures for the treatment of metastases during or after chemotherapy re-challenge was not recorded but may have been an impactful factor on clinical outcomes. It may be useful to also consider the re-challenge of monoclonal antibodies in subsequent analyses. Future studies should focus on the direct comparison of chemotherapy re-challenge to approved therapies such as regorafenib or trifluridine-tipiracil. Examination of pharmacoeconomic value benefit should also be measured in future investigations considering the sizeable differences in cost (Table 5) between therapies used in refractory mCRC (24).
Conclusions
Oxaliplatin or irinotecan-based re-challenge is a viable option as a third or fourth line treatment in select patients with mCRC. CBR and especially TTP compare favorably to approved third line therapies such as regorafenib or trifluridine-tipiracil.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by institutional review board of Banner Health (IRB00005094) and informed consent was waived by the IRB for this study as it was a retrospective chart review.
References
- American Cancer Society: Colorectal Cancer: Catching it early. Atlanta, GA: American Cancer Society, 2016. Available online: http://www.cancer.org/acs/groups/content/@marketing/documents/image/acspc-047433.pdf. Accessed electronically September 10, 2016
- National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) Program. Available online: http://www.seer.cancer.gov. Electronically accessed September 10, 2016.
- National Comprehensive Cancer Network. 2016. NCCN clinical practice guidelines in oncology™: Colon cancer. Version 2.2016. Jenkintown, PA.
- André T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 2009;27:3109-16. [Crossref] [PubMed]
- Barone C, Nuzzo G, Cassano A, et al. Final analysis of colorectal cancer patients treated with irinotecan and 5-fluorouracil plus folinic acid neoadjuvant chemotherapy for unresectable liver metastases. Br J Cancer 2007;97:1035-9. [Crossref] [PubMed]
- Grothey A, Sargent D, Goldberg RM, et al. Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil. leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol 2004;22:1209-14. [Crossref] [PubMed]
- Grothey A, Sargent D. Overall survival of patients with advanced colorectal cancer correlates with availability of fluorouracil, irinotecan, and oxaliplatin regardless of whether doublet or single-agent therapy is used first line. J Clin Oncol 2005;23:9441-2. [Crossref] [PubMed]
- Hochster HS, Hart LL, Ramanathan RK, et al. Safety and efficacy of oxaliplatin and fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer: results of the TREE Study. J Clin Oncol 2008;26:3523-9. [Crossref] [PubMed]
- Loupakis F, Cremolini C, Masi G, et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of metastatic colorectal cancer: results of the phase III randomized TRIBE trial. J Clin Oncol 2012;30:abstr 3510.
- Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 2004;351:337-45. [Crossref] [PubMed]
- Douillard JY, Siena S, Cassidy J, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol 2010;28:4697-705. [Crossref] [PubMed]
- Díaz-Rubio E, Gómez-España A, Massutí B, et al. First-line XELOX plus bevacizumab followed by XELOX plus bevacizumab or single-agent bevacizumab as maintenance therapy in patients with metastatic colorectal cancer: the phase III MACRO TTD study. Oncologist 2012;17:15-25. [Crossref] [PubMed]
- Chibaudel B, Maindrault-Goebel F, Lledo G, et al. Can chemotherapy be discontinued in unresectable metastatic colorectal cancer? The GERCOR OPTIMOX2 Study. J Clin Oncol 2009;27:5727-33. [Crossref] [PubMed]
- Strickler JH, Hurwitz H. Maintenance therapy for First-line Metastatic Colorectal Cancer: Activity and Sustainability. Oncologist 2012;17:9-10. [Crossref] [PubMed]
- Foo J, Michor F. Evolution of acquired resistance to anti-cancer therapy. J Theor Biol 2014;355:10-20. [Crossref] [PubMed]
- Hammond WA, Swaika A, Mody K, et al. Pharmacologic resistance in colorectal cancer: a review. Ther Adv Med Oncol 2016;8:57-84. [Crossref] [PubMed]
- Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:303-12. [Crossref] [PubMed]
- Mayer RJ, Van Cutsem E, Falcone A, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med 2015;372:1909-19. [Crossref] [PubMed]
- D’Alpino Peixoto R, Kumar A, Lim HJ, et al. Palliative oxaliplatin-based chemotherapy after exposure to oxaliplatin in the adjuvant setting for colon cancer. J Gastrointest Oncol 2015;6:487-91. [PubMed]
- Tonini G, Imperatori M, Vincenzi B, et al. Rechallenge therapy and treatment holiday: different strategies in management of metastatic colorectal cancer. J Exp Clin Cancer Res 2013;32:92-100. [Crossref] [PubMed]
- Townsend AR, Bishnoi S, Broadbridge V, et al. Rechallenge with oxaliplatin and fluoropyrimidine for metastatic colorectal carcinoma after prior therapy. Am J Clin Oncol 2013;36:49-52. [Crossref] [PubMed]
- Tournigand C, Cervantes A, Figer A, et al. OPTIMOX1: a randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-go fashion in advanced colorectal cancer - A GERCOR study. J Clin Oncol 2006;24:394-400. [Crossref] [PubMed]
- Suenaga M, Mizunuma N, Matsusaka S, et al. Phase II study of reintroduction of oxaliplatin for advanced colorectal cancer in patients previously treated with oxaliplatin and irinotecan: RE-OPEN study. Drug Des Devel Ther 2015;9:3099-108. [Crossref] [PubMed]
- Medicare program; Part B drug payment model. Washington, DC: Federal Register, 2017. Available online: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Part-B-Drugs/McrPartBDrugAvgSalesPrice/2017ASPFiles.html
- Costa T, Nuñez J, Felismino T, et al. REOX: Evaluation of the efficacy of retreatment with an oxaliplatin-containing regimen in metastatic colorectal cancer: a retrospective single-center study. Clin Colorectal Cancer 2017;16:316-23. [Crossref] [PubMed]