Viral hepatitis associated hepatocellular carcinoma outcomes with yttrium-90 radioembolization
Introduction
In 2015, approximately 35,660 individuals were diagnosed with hepatocellular carcinoma (HCC) and 24,550 died of their disease in the United States (1). Cirrhosis of any etiology may lead to HCC, but persistent viral infection with hepatitis B (HBV) or hepatitis C (HCV) accounts for over 80% of HCC cases worldwide (2). The incidence of HCC is different depending on the cause of cirrhosis and HCV is associated with the highest incidence (3).
Little is known regarding differences in post-treatment outcomes by underlying cause of HCC. Recent reports suggest that viral associated (VA) malignancies may be associated with better outcomes, such as improved locoregional control and survival as has been reported with chemoradiation for human papillomavirus (HPV) associated oropharynx cancer (4). This has led to national trials investigating dose de-escalation of these virally associated squamous cell cancers to reduce treatment associated toxicity with equivalent treatment outcomes.
Current management options for primary HCC, however, do not consider etiology. Regardless of the underlying cause of liver disease, patients are treated uniformly with surgical management as the standard of care. Unfortunately, most patients are not surgical candidates due to late stage presentation. Patients who are not eligible for surgical resection and have liver predominant disease are thus referred for nonsurgical locoregional interventions, which include percutaneous ablative techniques, stereotactic body radiation therapy (SBRT), transarterial chemoembolization (TACE), standard dose external beam radiation therapy, and/or yttrium-90 radioembolization (Y-90 RE). The choice of locoregional treatment mostly depends on tumor size and location, but also on the available treatment modality and expertise at an individual institution.
The aim of our current study was to investigate the outcomes of patients with unresectable HCC based on viral status (HBV and/or HCV) treated with Y-90 RE which we hypothesize will be different.
Methods
After IRB approval, 99 patients (122 treated lobes) with multifocal HCC were identified that were treated with lobar Y-90 RE at a single institution between January 1, 2009 and December 31, 2014. The decision to treat patients with Y-90 RE was made after presentation at our weekly multidisciplinary gastrointestinal tumor board which included members from interventional radiology, radiation oncology, medical oncology, surgical oncology, pathology, and radiology. Pretreatment patient evaluation included the patient’s medical history, physical exam, labs, and cross sectional imaging. HCC was diagnosed by biopsy and/or imaging characteristics that were consistent with the noninvasive criteria for HCC diagnosis (5). Chart review captured previous treatments, viral hepatitis status, α-fetoprotein values (AFP), Child-Pugh class (CP), albumin-bilirubin score (ALBI) (6), portal vein thrombosis (PVT), liver volume treated and doses delivered.
All patients underwent treatment planning angiography and technetium-99m macroaggregated albumin scanning to determine candidacy for Y-90 RE 10–14 days before the Y-90 RE treatment. Patients were treated with Y-90 glass microspheres (TheraSphere; Ottawa, Ontario, Canada). Dosimetry was based on volume of the liver lobe being treated. This brachytherapy device is approved by the Food and Drug Administration for HCC with or without PVT.
Patients returned for follow-up 4–6 weeks post treatment with imaging and labs followed by a 2–3 months surveillance interval once all disease was treated. mRECIST was applied to post treatment scans (7). Patients received additional treatment at the discretion of the treatment team at the time of failure based on imaging and AFP. Additional treatments consisted of sorafenib, Y-90 RE, TACE, or SBRT based on a multidisciplinary approach. No patients underwent resection or transplantation following treatment.
Factors associated with viral hepatitis status [VA HCC vs. non-VA (NVA) HCC] were compared via Pearson’s Chi-square and Mann-Whitney U test on univariate analysis (UVA). Variables that were associated with viral status on UVA (P<0.1) were included in a multivariate logistic regression (MVA) to identify factors independently associated with viral hepatitis status. Pearson’s Chi-square was also utilized in comparing liver decompensation, defined by recurrent ascites and/or encephalopathy, between VA HCC and NVA HCC.
Intrahepatic control (IHC) was defined by HCC recurrence in the treated liver. Extrahepatic control (EHC) was defined by HCC regional or distant progression. Progression free survival (PFS) was defined by the time to first recurrence or death from any cause. Overall survival (OS) was defined to the time of death from any cause or last contact. Time-to-event was defined as time duration from the day of Y-90 RE treatment to event. IHC, EHC, PFS, and OS were calculated according to the Kaplan-Meier method and survival differences were assessed via the log-rank test. Prognostic factors with P<0.1 for any outcome measure, in addition to continuous variables (age at diagnosis, pretreatment AFP, volume treated, and dose), were included in a multivariate Cox proportional hazard regression analysis. Two-sided P values at the level of significance of <0.05 were considered statistically significant. All analyses were performed using SPSS version 22 (IBM, Armonk, NY, USA).
Results
Patient demographics, tumor characteristics, and treatments are presented in Table 1. The median age of patients was 69 years (range, 45–86 years). The majority of patients were male (82%, n=81), had 1 lobe treated (76%, n=75), were CP A (91%, n=90), had no PVT (90%, n=89), and had local disease (96%, n=95).
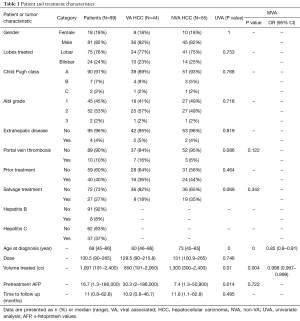
Full table
A total of 44 patients (44%) had known viral hepatitis (HBV and/or HCV) with the majority having hepatitis C. Table 1 depicts the differences between the VA hepatitis HCC patients and the NVA hepatitis HCC patients. Median follow up for VA and NVA hepatitis patients was 10.9 months (range, 0.8–46.7 months) and 11.8 months (range, 1.1–62.8 months), respectively. On multivariate logistic regression analysis, patients with VA HCC were younger (60 vs. 73 years of age, P<0.001) and had smaller pretreatment liver volumes (850 vs. 1,300 cc, P<0.001); however, there was no difference with respect to gender, pre-treatment AFP, CP, ALBI, PVT, extrahepatic disease, previous treatment, and dose given on MVA. Median dose for VA and NVA hepatitis patients was 129.5 Gy (range, 90–215.8 Gy) and 131 Gy (range, 100.9–265 Gy), respectively (P=0.75).
Median time to any intrahepatic progression for all patients, VA HCC, and NVA HCC was 11.3, 26.3, and 7.4 months, respectively. The 1-year IHC was 67% for VA HCC versus 34% for NVA HCC (P=0.067) (Figure 1A). On MVA, improved IHC was seen in males (HR 0.4, 95% CI: 0.18–0.9, P=0.026), whereas older patients (HR 1.05, 95% CI: 1.02–1.09, P=0.003), larger volumes treated (HR 1.001, 95% CI: 1–1.002, P=0.006), and extrahepatic disease at presentation (HR 26.43, 95% CI: 7.54–92.65, P<0.001) were associated with an IHC detriment (Table 2). Median time to any extrahepatic failure for all patients and for NVA HCC was not reached but was 24 months for VA HCC. The 1-year EHC was 63% for VA HCC versus 86% for NVA HCC (P=0.029) (Figure 1B). On MVA, patients with VA HCC (HR 2.74, 95% CI: 1.12–6.67, P=0.027), extrahepatic disease at presentation (HR 135.7, 95% CI: 21.43–859.27, P<0.001), and larger volumes of parenchymal liver treated (HR 1.001, 95% CI: 1–1.002, P=0.035) were associated with a detriment to EHC (Table 2).

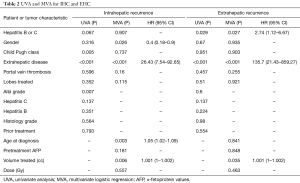
Full table
Median PFS for all patients, VA HCC, and NVA HCC was 8.2, 10.4, and 7.1 months, respectively, with a median OS for all patients, VA HCC, and NVA HCC of 12.9, 11.3, and 16.5 months, respectively. The 1-year progression free survival was 45% for VA HCC versus 31% for NVA HCC (P=0.56) with a 1-year OS of 46% for VA HCC versus 55% for NVA patients (P=0.55). On MVA, worse OS was observed in patients that were older (HR 1.04, 95% CI: 1.01–1.07, P=0.012), CP B/C (HR 4.27, 95% CI: 1.55–11.76, P=0.005), had PVT (HR 2.17, 95% CI 1.01–4.64, P=0.047), or had extrahepatic disease at presentation (HR 15.25, 95% CI: 4.53–51.35, P<0.001). Patients that underwent salvage treatment which included sorafenib, Y-90 RE, TACE, or SBRT for a recurrence had improvement in OS (HR 0.35, 95% CI: 0.19–0.66, P=0.001), whereas gender, viral hepatitis, pre-treatment AFP, volume treated, and dose received were not statistically significant on MVA.
After the Y-90 RE treatment a total of 18 patients (18%) had developed decompensated liver failure based on recurrent ascites and/or encephalopathy. These patients were medically managed. There was no difference between VA HCC (18%, n=8) and NVA HCC (18%, n=10) (P=0.99). The majority of patients that died did so without liver decompensation (79%, n=51).
Discussion
The majority (75%) of HCC cases worldwide are VA, with other causes including alcoholic cirrhosis, non-alcoholic steatohepatitis (NASH), and other less common causes (8). The presumed pathogenesis to HCC from each etiology might not be the same. HBV contains a partially double stranded genomic DNA that is thought to be carcinogenic in an indirect fashion since it doesn’t encode a dominant oncogene. Therefore HBV may lead to HCC through activation of oncogenes and induction of genetic instability by HBV DNA integration or regulatory HBV protein X (8). In contrast, HCV is a single stranded, mostly cytoplasmic, RNA virus and is likely to predispose the liver to cancer through alteration of cell signaling and metabolism leading to chronic inflammation and oxidative stress (9). There is also suggestion that the HCV core protein and several other HCV proteins have been shown to have a direct oncogenic effect (10). Other studies have shown interaction with various regulators of the cell cycle including p53, retinoblastoma pathway, and possibly DEAD box protein 5 and 3 (DDX5 and DDX3) further promoting carcinogenesis (11). Both HBV and HCV are associated with modulation of the host immune response further leading to an increased risk of malignancy (8). Lastly, NASH leading to HCC is thought to be due to a chronic excess of nutrients causing endoplasmic reticulum stress, steatosis, and insulin resistance which leads to inflammation and an environment that favors carcinogenesis (10). Each etiology promoting liver cirrhosis and HCC has a different mechanism and therefore response and outcomes to various treatments may be different.
There are limited data available on outcomes based on etiology of cirrhosis and development of HCC. Chin et al, reported no difference in outcomes in patients treated with TACE regardless of viral status (12). They report on 201 patients whose 1st treatment was TACE and found improvement in OS in patients with response to treatment, early BCLC stage, CP A, and tumor size <4 cm. In contrast, Chen et al. reported on patients treated with resection, TACE, percutaneous ethanol injection, and best supportive care and found differences in outcomes based on viral etiology (13). Patients with HCV associated HCC had improved survival in the first five years regardless of treatment but worse at 10 years. They also reported improved initial 5-year OS in HCV associated HCC when treated with TACE or percutaneous ethanol injection as well as the cohort with best supportive care. They conclude that patients with HCV may have a higher rate of disease recurrence and/or worsening liver function long term and therefore the OS benefit is lost by 10 years. Tandoi et al. reported that patients with HCV had worse outcomes and increased disease recurrence when treated with orthotopic liver transplant (14).
We report the first study looking at outcomes based on VA HCC versus NVA HCC patients treated with Y-90 RE. We found a trend to improved IHC in patients treated with Y-90 RE who had VA HCC. This is in concordance with the improved local regional outcomes of patients with VA (HPV+) oropharynx squamous cell carcinoma treated with radiotherapy.
Interestingly, we found a worse EHC in patients with VA HCC. This isn’t clear as far as the cause of worsening EHC but explains why there are no differences in PFS or OS. One explanation is undiagnosed micrometastatic disease at presentation. There are limited systemic options for patients with metastatic or unresectable advanced HCC. In the Sorafenib Hepatocellular Carcinoma Assessment Randomized Protocol (SHARP) trial, patients with unresectable HCC with CP A treated with 1st line sorafenib versus placebo showed an increase in survival from 7.9 to 10.7 months (15). A subgroup analysis of the SHARP trial reported by Llovet et al. looked at outcomes based on etiology (HCV, HBV, alcoholic associated) (16). The authors reported differences in outcomes based on HCV, HBV and alcohol related HCC. They found that patients with HCV associated HCC treated with sorafenib had improved median OS (14 versus 7.4 months) and improved disease control rate while the HBV associated HCC treated with sorafenib had improved median OS (9.7 versus 6.1 months) but shorter median time to progression. This shows that the etiology leading to HCC may play a role in treatment response and outcomes.
This study is limited by the small sample size as well as the majority of the VA patients being HCV positive. This is a retrospective review and therefore has inherent bias. There are many genotypes of HCV but unfortunately these data were not available nor was the viral load pre and post treatment. Due to the small number of HBV patients, we combined all VA HCC’s together in our analysis; however, when removing the HBV patients the outcomes were similar (data not shown).
HCC treatment response and natural history may vary based on viral association and there may be a benefit to personalizing treatment based on etiology of liver disease leading to HCC. Future research should include evaluation of systemic therapy following local therapy in VA HCC given the increased rate of regional and distant failure. HBV and HCV are both associated with immune modulation and therefore immunotherapy may play a key role in HBV and HCV associated HCC to improve outcomes from this deadly disease.
In our study of patients with HCC treated with Y-90 RE, outcomes are different based on the etiology of cirrhosis leading to malignancy. Patients with VA HCC had a trend to improved IHC and significantly worse EHC. Consideration should be made for early systemic therapy following Y-90 RE in patients with viral hepatitis associated HCC to better control extrahepatic progression.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical statement: This study was approved by Institutional Review Board of Moffitt Cancer Center (No. MCC 16567) and informed consent was taken from all the patients.
References
- American Cancer Society. Cancer Facts and Figures 2015. Atlanta, Ga: American Cancer Society, 2015.
- Bosch FX, Ribes J, Borras J. Epidemiology of primary liver cancer. Semin Liver Dis 1999;19:271-85. [Crossref] [PubMed]
- Fattovich G, Stroffolini T, Zagni I, et al. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology 2004;127:S35-50. [Crossref] [PubMed]
- Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 2010;363:24-35. [Crossref] [PubMed]
- Peterson MS, Baron RL, Marsh JW Jr, et al. Pretransplantation surveillance for possible hepatocellular carcinoma in patients with cirrhosis: epidemiology and CT-based tumor detection rate in 430 cases with surgical pathologic correlation. Radiology 2000;217:743-9. [Crossref] [PubMed]
- Toyoda H, Lai PB, O'Beirne J, et al. Long-term impact of liver function on curative therapy for hepatocellular carcinoma: application of the ALBI grade. Br J Cancer 2016;114:744-50. [Crossref] [PubMed]
- Kim MN, Kim BK, Han KH, et al. Evolution from WHO to EASL and mRECIST for hepatocellular carcinoma: considerations for tumor response assessment. Expert Rev Gastroenterol Hepatol 2015;9:335-48. [Crossref] [PubMed]
- Bartosch B. Hepatitis B and C viruses and hepatocellular carcinoma. Viruses 2010;2:1504-9. [Crossref] [PubMed]
- de Oliveria Andrade LJ, D'Oliveira A, Melo RC, et al. Association between hepatitis C and hepatocellular carcinoma. J Glob Infect Dis 2009;1:33-7. [Crossref] [PubMed]
- Bartosch B, Thimme R, Blum HE, et al. Hepatitis C virus-induced hepatocarcinogenesis. J Hepatol 2009;51:810-20. [Crossref] [PubMed]
- McGivern DR, Lemon SM. Tumor suppressors, chromosomal instability, and hepatitis C virus-associated liver cancer. Annu Rev Pathol 2009;4:399-415. [Crossref] [PubMed]
- Chen BB, Shih IL, Wu CH, et al. Comparison of characteristics and transarterial chemoembolization outcomes in patients with unresectable hepatocellular carcinoma and different viral etiologies. J Vasc Interv Radiol 2014;25:371-8. [Crossref] [PubMed]
- Chen CH, Huang GT, Yang PM, et al. Hepatitis B- and C-related hepatocellular carcinomas yield different clinical features and prognosis. Eur J Cancer 2006;42:2524-9. [Crossref] [PubMed]
- Tandoi F, Ponte E, Saffioti MC, et al. Liver transplantation for hepatocellular carcinoma within Milan Criteria in patients with Model for End-Stage Liver Disease score below 15: the impact of the etiology of cirrhosis on long-term survival. Transplant Proc 2013;45:2711-4. [Crossref] [PubMed]
- Rimassa L, Santoro A. Sorafenib therapy in advanced hepatocellular carcinoma: the SHARP trial. Expert Rev Anticancer Ther 2009;9:739-45. [Crossref] [PubMed]
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. [Crossref] [PubMed]


