Oxaliplatin-induced bronchiolitis obliterans organizing pneumonia following hyperthermic intraperitoneal chemotherapy
Introduction
Oxaliplatin, a third-generation platin derivative, acts via interruption of DNA synthesis (1). This drug has demonstrated activity in patients with resected colorectal cancer as well as for patients with stage IV colorectal cancer (2). It is the most commonly used chemotherapeutic agent for hyperthermic intraperitoneal chemotherapy (HIPEC) (3). The main secondary reactions related to oxaliplatin are hematologic effects, GI effects, and neurologic toxicity (4).
A less common side effect is pulmonary toxicity, mainly characterized by interstitial pneumonitis or bronchiolitis obliterans organizing pneumonia (BOOP). The exact incidence of this side effect is unknown, as well as the risk factors, but the pulmonary toxicity can be fatal (5). All the cases described in the English literature have occurred when using oxaliplatin by IV route (6).
To our knowledge, we report here the first case of oxaliplatin induced BOOP following HIPEC. Informed consent of our patient was obtained to right this report.
Case presentation
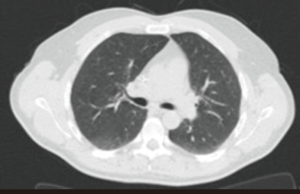
A 47-year-old male, never smoker, with a familial adenomatous polyposis, was diagnosed with a non-metastatic colon adenocarcinoma, in May 2014. First, a Hartmann’s procedure with colostomy was performed. The tumour was locally advanced with an abscess signing a tumoral perforation (ypT4N1M0). Chemotherapy by FOLFOX protocol (5-fluorouracil/oxaliplatin/folinic acid) was started. After six infusions, oxaliplatin was stopped due to recurrent severe trismus after each infusion. The received total dose of oxaliplatin was 680 mg/m2. At the end of the 12 planned cycles of chemotherapy, the patient had no signs of disease on abdominal CT-scan. Chest CT-scan was normal (Figure 1).
The patient was included in the ProphyloCHIP trial (7). This trial compares observation (standard arm) to a hyperthermic intraperitoneal chemotherapy (experimental arm) in patients with colorectal cancer initially treated by surgery and adjuvant chemotherapy and with a high probability to develop a peritoneal carcinomatosis. The patient was randomized in the experimental arm. During surgery, a peritoneal carcinomatosis was discovered. An omentectomy, a cholecystectomy, a proctectomy, a partial cystectomy and a total colectomy were performed. Then, an intravenous infusion of 784 mg of 5-FU and 39 mg of folinic acid during 1 hour was performed, followed by a hyperthermic intraperitoneal chemotherapy with 588 mg of oxaliplatin and 392 mg of CPT-11 (irinotecan) at 42.5 °C during 30 minutes. The procedure was uneventful.
Two days after the HIPEC, the patient’s respiratory status rapidly deteriorated, without fever, and required non-invasive ventilation. The chest CT-scan showed diffuse bilateral interstitial infiltrates and no pulmonary emboli (Figure 2). The bronchial fibroscopy was considered as unsafe. The microscopic examination of sputum was inflammatory, with a lot of macrophages. The bacteriologic examination of sputum was negative, as well as the search for Legionella urinary antigen. Brain natriuretic peptide was in the normal range.
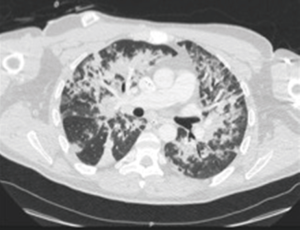
A diagnosis of BOOP was then suspected.
The patient was treated with high-dose of corticosteroids (1 mg/kg) and large spectrum antibiotics and antifungal drugs, until the diagnosis of bacterial infection was ruled out.
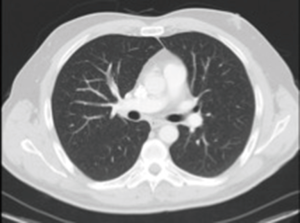
Twenty-four hours after starting the infusion of corticosteroids, the patient’s respiratory status dramatically improved. A new chest CT-scan performed five days after the beginning of corticosteroids showed a major improvement of the pulmonary infiltrates (Figure 3). The corticoids were gradually tapered on six weeks, without relapse.

Five months later, the patient had no relapse of the BOOP and the chest CT-scan showed a total recovery (Figure 4).
Discussion
Different diagnoses of lung infiltrates in cancer patients following chemotherapy include infections, heart failure, neoplasia, pulmonary bleeding and drug toxicity. Drug-induced pneumonitis is usually a diagnosis of exclusion. In our case, we were not able to obtain bronchoalveolar lavage (BAL) fluid due to severe respiratory failure. However, there was a temporal relationship between progressive dyspnea and the chemotherapy, which suggests that chemotherapy was the causative agent. Moreover, the rapid and sustained improvement achieved with high-dose steroid therapy in the absence of any signs of infectious disease permits the conclusion to be drawn that the pulmonary opacities had been caused by a drug-induced reaction.
Respiratory side effects following 5-FU administration are very uncommon (8). The responsibility of CPT-11 for pulmonary reaction is unlikely because CPT-11 has been less frequently involved in drug toxicity. Moreover, interstitial fibrosis has been more frequently described than BOOP with this agent (9).
Then, we considered that the most likely cause of BOOP in our patient was oxaliplatin.
Early safety studies with oxaliplatin have found no significant increase in pulmonary complications, except for the dyspnoea that may have occurred in the setting of a hypersensitivity reaction (10). Several cases of cold-triggered acute inspiratory stridor lasting from a few seconds up to some minutes have been described immediately after infusion of oxaliplatin (11). In 2001, Trisolini et al. reported the first case of interstitial pneumonia induced by oxaliplatin (12). Since then, different patterns of oxaliplatin-induced lung injury have been reported (8). Garrido et al. reported the first case of oxaliplatin-induced BOOP in 2007 (13). Cryptogenic organising pneumonia (COP) seems now to be one of the most common forms of oxaliplatin-induced respiratory disease (excepting inspiratory stridor).
The clinical presentation of BOOP is variable, ranging from almost asymptomatic transient infiltrates to acute respiratory failure. Symptoms are nonspecific (dyspnoea, cough, fever). Multiple alveolar opacities on imaging represent the most frequent and typical imaging features of COP. These are usually bilateral and peripheral, and are often migratory (14). BAL usually shows a mixed pattern at differential cell count consisting of an increase in lymphocytes, neutrophils and eosinophils. Organising pneumonia is defined histopathologically by intra-alveolar buds of granulation tissue, consisting of intermixed myofibroblasts and connective tissue (14).
Few risk factors have been proposed at this point. Twenty-six cases of oxaliplatin-related pulmonary toxicity have been described in the English literature in 2015 (6). Sixteen of these cases (61.5%) were fatal. Most patients were males (20/26, 77%), older than 60 years (24/26, 92.3%), had a metastatic colorectal carcinoma (16/26, 61.5%), and were treated with oxaliplatin for less than 6 months (20/26, 76%). Seven of these 26 (27%) patients had previous lung disease, 2 (8%) were smokers and 4 (15%) had hypertension. The mean administered dose of oxaliplatin before the interstitial pneumonia was about 650 mg/m2 and the median dose was 510 mg/m2. However, the doses are highly variable, ranging from 100 to 1,200 mg/m2 (6). Our patient received 680 mg/m2 of oxaliplatin before HIPEC, which is close to the mean dose found in the literature.
The mechanism for this pulmonary injury is not yet determined. However, there is data suggesting that oxaliplatin may cause glutathione depletion, which could be involved in the pathogenesis of liver damage caused by the drug. In the lung, glutathione plays a significant role as protector against oxidative damage, and depletion caused by oxaliplatin could be the factor triggering the pulmonary lesions leading to interstitial pneumonitis and subsequent pulmonary fibrosis (15). There is no adequate data suggesting whether treatment with antioxidant agents, such as N-acetyl cysteine, which may replenish glutathione deposits, may be beneficial. There is no standard treatment so far. High-dose corticosteroid treatment is commonly given for serious cases of this potentially lethal complication.
The unusual feature of this case was that pneumonitis developed after intraperitoneal administration of oxaliplatin. Despite of a large molecular weight, systemic absorption of oxaliplatin is possible, as confirmed by our case report. According to Elias’ study (16), systemic absorption of oxaliplatin administered by intraperitoneal route is about 50% (16), which is consistent with the occurrence of systemic side effects. To our knowledge, this is the first case of oxaliplatin-induced COP following hyperthermic intraperitoneal chemotherapy. We then wanted to bring this uncommon, but potentially severe oxaliplatin-related pulmonary complication to the attention of treating oncologists as well as of surgical oncological surgeons and anesthetists.
Conclusions
This case demonstrates for the first time that oxaliplatin delivering during a HIPEC can be a cause of BOOP, which has been more widely described after intravenous infusions. Its evolution can be potentially fatal and the treatment by high dose of corticosteroids could be effective, especially if it is early prescribed. Thus, it is important to know this diagnosis when we face a patient with respiratory symptoms after a HIPEC with oxaliplatin.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Raymond E, Faivre S, Chaney S, et al. Cellular and molecular pharmacology of oxaliplatin. Mol Cancer Ther 2002;1:227-35. [PubMed]
- de Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 2000;18:2938-47. [Crossref] [PubMed]
- Elias D, Honoré C, Dumont F, et al. Results of systematic second-look surgery plus HIPEC in asymptomatic patients presenting a high risk of developing colorectal peritoneal carcinomatosis. Ann Surg 2011;254:289-93. [Crossref] [PubMed]
- Cassidy J, Misset JL. Oxaliplatin-related side effects: characteristics and management. Semin Oncol 2002;29:11-20. [Crossref] [PubMed]
- Arévalo Lobera S, Sagastibeltza Mariñelarena N, Elejoste Echeberría I, et al. Fatal pneumonitis induced by oxaliplatin. Clin Transl Oncol 2008;10:764-67. [Crossref] [PubMed]
- Moskovitz M, Wollner M, Haim N. Oxaliplatin-Induced Pulmonary Toxicity in Gastrointestinal Malignancies: Two Case Reports and Review of the Literature. Case Rep Oncol Med 2015;2015:341064. [Crossref] [PubMed]
- National Institutes of Health : Trial Comparing Simple Follow-up to Exploratory Laparotomy Plus “in Principle” (Hyperthermic Intraperitoneal Chemotherapy) HIPEC in Colorectal Patients (ProphyloCHIP), NCT01226394.
- Pneumotox: Foucher P, Camus P. Pneumotox®. 1997. Available online: (accessed September 2002).http://www.pneumotox.com
- Yoshii N, Suzuki T, Nagashima M, et al. Clarification of clinical features of interstitial lung disease induced by irinotecan based on postmarketing surveillance data and spontaneous reports. Anticancer Drugs 2011;22:563-8. [Crossref] [PubMed]
- Pasetto LM, Monfardini S. Is acute dyspnea related to oxaliplatin administration? World J Gastroenterol 2006;12:5907-8. [Crossref] [PubMed]
- Polyzos A, Tsavaris N, Gogas H, et al. Clinical features of hypersensitivity reactions to oxaliplatin: a 10-year experience. Oncology 2009;76:36-41. [Crossref] [PubMed]
- Trisolini R, Lazzari Agli L, Tassinari D, et al. Acute lung injury associated with 5-fluorouracil and oxaliplatinum combined chemotherapy. Eur Respir J 2001;18:243-5. [PubMed]
- Garrido M, O’Brien A, González S, et al. Cryptogenic organizing pneumonitis during oxaliplatin chemotherapy for colorectal cancer: case report. Chest 2007;132:1997-9. [Crossref] [PubMed]
- Cordier JF. Cryptogenic organising pneumonia. Eur Respir J 2006;28:422-46. [Crossref] [PubMed]
- Rubbia-Brandt L, Audard V, Sartoretti P, et al. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol 2004;15:460-6. [Crossref] [PubMed]
- Elias D, Bonnay M, Puizillou JM, et al. Heated intra-operative intraperitoneal oxaliplatin after complete resection of peritoneal carcinomatosis: pharmacokinetics and tissue distribution. Ann Oncol 2002;13:267-72. [Crossref] [PubMed]