Characterization of malignant gastrointestinal stromal tumors—a single center experience
Introduction
Although gastrointestinal stromal tumors (GISTs) account for less than 1% of all gastrointestinal (GI) tumors and 5% of all sarcomas (1), they are the most common mesenchymal neoplasia of the GI tract (2) and affect approximately 3,000–4,000 people each year in the US, with a median patient age of 60 years (3). Most of these tumors are localized to the stomach (60%) or jejunum and ileum (30%); fewer are detected in the duodenum and the colon rectum, and these tumors are very rarely found in the esophagus (4). They may exhibit malignant behavior in 20–25% or 40–50% of cases, depending on whether they are localized to the stomach or the small intestine, respectively (4). They typically metastasize to the abdominal cavity or liver; metastasis to other parenchymatous organs is rare, and metastasis to the lymph nodes or lungs is even less common (4). It is likely that GISTs arise from the interstitial cells of Cajal, KIT-positive and KIT ligand-positive and dependent cells that are located around the myenteric plexus and the muscularis propria throughout the GI tract (4,5). Approximately 80–95% of GISTs harbor mutations in the KIT proto-oncogene (5), and 3–5% have a gain-of-function mutation in the platelet-derived growth factor receptor-alpha (PDGFR-α) gene (6).
The management of GISTs has changed over the last 10 years with the introduction of the first tyrosine kinase inhibitor (7). Although many new drugs are currently under evaluation and alternative activated signaling pathway treatments have been discovered, surgery remains the only potentially curative remedy for primary localized GISTs (8) and the only curative option for locally advanced or recurrent GISTs. Unfortunately, the recurrence rate after surgery alone is as high as 50% at 5 years (9), and more than 50% of high-risk patients will develop a recurrence within 2 years (10). Postoperative adjuvant chemotherapy has not generally been recommended because conventional cytotoxic agents are ineffective against such tumors (11). Recently, a randomized, double-blind control trial by Dematteo et al. (12) stated that 1-year adjuvant therapy with imatinib improves recurrence-free survival after complete resection of primary GIST (98% vs. 83% at 1 year, P<0.0001). However, many high-risk patients suffered relapses 6 months after completing adjuvant treatment (12).
The recurrence rate, which is related to the unpredictable behavior of these tumors, continues to be a major topic of investigation. Several studies have investigated the main resultant predictors (9,13-15). Some factors are widely recognized as being predictive of recurrence, and several risk stratification scales have been proposed (2,16-20). Despite these risk assessments, approximately 10% of GISTs have an unexpected course, suggesting that many other factors affect their behavior.
Hence, we retrospectively evaluated our results for localized and locally advanced GISTs to assess the predictive variables of disease recurrence.
Methods
Between September 2004 and January 2011, the clinical and pathological data for all patients who underwent surgery for localized and advanced GISTs were collected from an institutional GIST database that was prospectively maintained in our Department of Surgery. Preoperative clinical assessments were based on upper gastrointestinal endoscopy, endoscopic ultrasound (EUS) and CT examinations of the thorax, abdomen and pelvis. EUS-guided fine-needle aspiration (FNA) was not performed on any of the study patients.
Laparotomy was preferred to the laparoscopic approach in cases where the tumor size exceeded 8 cm or for patients with medical history significant for multiple abdominal operations, depending upon the anatomical location of the stromal tumor. A laparoscopic procedure to open conversion was classified as any case in which laparoscopy was used with therapeutic intent with the subsequent creation of a laparotomy incision, regardless of the extent of the attempted resection.
Open resections were typically performed through bilateral sub-costal incisions for lesions that were localized in the supramesocolic region; midline incisions were chosen for resections of the colon and rectum. For the laparoscopic approach, entry into the peritoneum was achieved through the Hasson technique. Three or four port sites were generally used to facilitate the gastric resections. Gastric wedge resections were usually achieved using an endoGIA stapler, and the tumor specimens were extracted using an Endocatch bag. The tumor sizes were recorded as the largest diameter in any dimension of the primary tumor and were stratified as ≤2 cm, from >2 to ≤5 cm, from >5 to ≤10 cm and >10 cm. If a visible tumor was not resected or if its margins were grossly involved, the resection was coded as R2. Margins of resected specimens were analyzed for the presence of microscopic disease. If the margins were microscopically positive, the resection was coded as R1. If all disease was completely resected with tumor-free margins, the resection was coded as R0. Histological evaluations were always combined with immunohistochemical analysis and mutational sampling of the KIT and PDGFR-α genes. Histopathological sections were cut from paraffin blocks for routine hematoxylin/eosin and immunohistochemical staining using the following monoclonal and polyclonal antibodies: CD117, CD34, smooth muscle actin, desmin and S-100. Proliferative activity was evaluated by counting the number of mitoses per 50 high-power fields (HPFs). Genomic DNA from the tumor tissues was extracted from the paraffin-embedded tissue using a microdissection procedure and the QIAamp DNA FFPE Tissue Kit (QIAGEN, Inc., Alameda, CA, USA). Through polymerase chain reaction (PCR) amplification and direct sequencing, all cases were screened for activating KIT mutations within exons 9, 11, 13 and 17 and for PDGFR-α mutations within exons 12, 14 and 18.
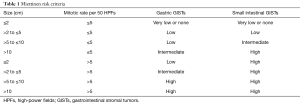
Primary tumor risk stratification was defined according to Miettinen criteria (Table 1) (16). Firstline adjuvant therapy was performed in patients with intermediate- to high-risk GISTs, and advanced chemotherapy was employed with a recurrent disease prognosis according to National Comprehensive Cancer Network (NCCN) and European Society for Medical Oncology (ESMO) guidelines (21,22).
Full table
After surgery, all patients were seen at 1-month intervals for clinical interviews. Every 3 to 4 months, blood tests, physical examinations and CT scans of the thoracic, abdominal and pelvic regions were repeated until the 3rd year and every year thereafter until the 5th year for intermediate- and high-risk GISTs; they were performed every 6 months for 5 years after surgery for low-risk and very low-risk GISTs. Recurrences were recorded if they were detected during scheduled or unscheduled procedures. Unscheduled visits or procedures were planned in cases where new symptoms developed.
The time from operation to death or the date of the last observation was defined as the survival time. The disease-free interval was defined as the time from the date of the operation until the detection of the first confirmed recurrence or metastasis.
Statistical analysis
Medians, ranges and frequencies were used as descriptive statistics. Fisher’s exact test, the χ2 test, the linear by linear association test and the Mann-Whitney U-test were applied as appropriate.
The patient overall survival and disease-free survival rates were analyzed by the Kaplan-Meier method. A P value of less than 0.05 was considered significant. The receiver operating characteristic (ROC) analysis was used to determine the optimal cut-off value for tumor dimension to predict recurrences. Statistical analyses were performed using the SPSS v.13.00 software package (SPSS, Inc., Chicago, IL, USA).
Results
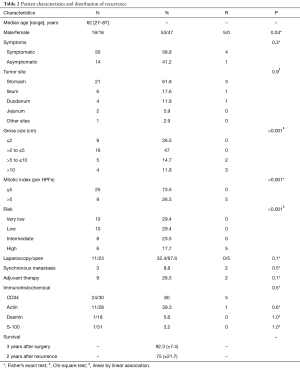
Between September 2004 and January 2011, 34 patients underwent operations for primary, localized and advanced GISTs. The patients’ clinicopathologic characteristics are summarized in Table 2. The patient group consisted of eighteen males and sixteen females, with a median age of 62 years (range, 27–87 years). The stomach was the most common primary tumor site (61.8%), followed by the ileum (17.6%), duodenum (11.8%), jejunum (5.9%) and pancreas (2.9%). We performed 21 laparotomies: 8 gastric wedge resections, 5 nodular excisions, 3 ileum resections, 2 total gastrectomies, 1 distal gastric resection and 2 pancreatoduodenectomies. A total of 11 laparoscopies (7 gastric wedge resections, 2 ileum resections and 2 nodular excisions of the duodenum and jejunum) were performed. Two laparoscopies to open conversions of two GISTs of the stomach were undertaken because of large tumor dimensions and intra-operative bleeding, respectively.
Full table
At the time of the operation, three patients had GIST-synchronous metastases located in the liver (23) or peritoneum.
The median tumor size was 3.1 cm (range, 1–35 cm); 3.2 cm (range, 2–7 cm) in the laparoscopic group and 3.0 cm (range, 1–35 cm) in the open group (P=0.57; Mann-Whitney U test). All lesions had negative resection margins. Radical resection was achieved in all 34 patients.
During the immunohistochemical analysis, CD117 positivity was detected in all patients (100%), and CD34 positivity was detected in 24 out of 30 (80%) patients. S-100, smooth-muscle actin and desmin positivity was rare, occurring in 1 out of 31 (3.2%), 11 out of 28 (39.3%) and 1 out of 18 (5.6%) patients, respectively. The Mitotic Index was >5 mitoses per 50 HPFs in 9 (26%) cases and ≤5 in 25 cases (74%).
Risk stratification was as follows: 6 patients (17.7%) had high-risk tumors, 8 (23.5%) had intermediate-risk tumors, 10 (29.4%) had low-risk tumors and 10 (29.4%) had very low-risk tumors.
The median hospitalization time was 11 days (range, 5–50 days). Laparoscopy was not associated with a significantly shorter median hospital stay: 10 (range, 5–17 days) vs. 12 days (range, 7–50 days) (P=0.09; Mann-Whitney U test). No perioperative mortality was observed. Two patients (one with a pancreatoduodenectomy and one with a gastric wedge resection) developed pancreatitis 8 and 15 days from operations that required re-intervention (the former required a total pancreatectomy, and the latter required a toilette of the abdominal cavity). One patient had pleural effusion and abdominal collection that was treated by a thoracic tube and surgical toilette. No patients of the laparoscopic group had perioperative complications.
The median follow-up (FU) was 20 months (range, 6–86 months). A first-line adjuvant therapy was performed in 9 patients: 1 withdrew from treatment owing to toxicity, and 3 patients were recruited to the observation control group of a phase III trial study (24).
Disease relapse occurred in 5 cases: 3 in the abdomen only, 1 in the abdomen and liver and 1 in the abdomen and pelvis. One patient underwent a re-intervention that consisted of an ileum resection associated with the Hartmann procedure.
The mutational analysis of the KIT and PDGFR-α genes is reported in Table 3. KIT mutations were found in 27 patients (79.4%): 25 cases (92.6%) involving exon 11 and 2 cases (7.4%) involving exon 9. PDGFR-α mutations were recorded in 5 patients (14.7%): 4 (80%) involving exon 18 and 1 (20%) involving exon 12; 2 cases (5.9%) were identified as wild type. All relapses were KIT exon 11 deletions within sequences c.1669_1674del (Trp557_ 223 Lys558del) and c.1684_1728 (Glu562_Leu576del).
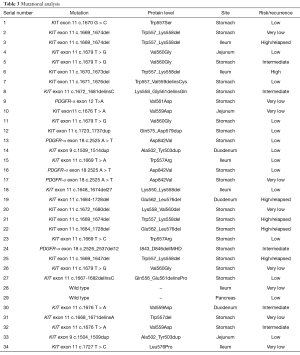
Full table
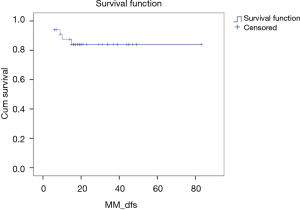
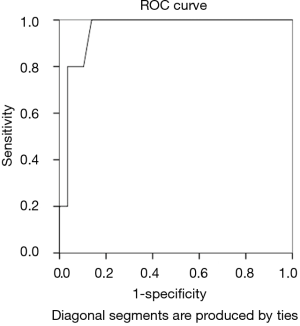
The recurrence-free survival rate was 87.5% (±5.9%) at 1 year. The disease-free survival is shown in Figure 1. All relapses occurred in high-risk patients (P<0.001). The distribution of recurrence by patients and the tumor features are reported in Table 2. Using the ROC curve (Figure 2), the optimal maximum value for the tumor size was 7 cm, with an area under the curve (AUC) of 0.955. The 3-year overall survival rate was 92.3% (±7.4%), and the 2-year survival rate after the diagnosis of recurrence was 75% (±21.7%).
Discussion
Throughout the GIST literature, validated predictive factors of recurrence and survival and, more recently in a retrospective study, tumor location, size and the mitotic index became independent predictors after complete resection (15). In our study, the tumor size and the mitotic index were the strongest predictive factors. The tumor site was not significant for statistical analysis, although it has been demonstrated that GISTs that arise from the small intestine have less favorable prognoses than gastric GISTs (15,16); the reason for this difference could be explained by the small number of relapses in this series. Further, by calculating the corresponding ROC curve, we found 7 cm to be the optimal tumor size threshold value. Accordingly, a tumor size of 7 cm was recently suggested as a threshold value for malignancy in gastric GISTs (25,26). In larger studies, the mitotic rate was a significant prognostic factor for all size groups of small intestinal GISTs (14), although few data are available for tumors smaller than 2 cm with high mitotic numbers. Rarer findings in larger studies indicate that 2–3% of small GISTs with low mitotic activity have an unexpected adverse outcome, while some very large tumors with low mitotic rates have relatively good prognoses (13). These seemingly unusual behaviors might be caused by different gene mutational profiles. Approximately 80–95% of GISTs carry KIT mutations (5), and 3–5% have a gain-of-function mutation in the PDGFR-α gene (6). In addition, gastric GISTs with exon 11 missense mutations have been associated with better prognoses than deletion/insertion or duplication mutations of the same exon (13). No similar difference was found for small intestinal GISTs (4). Rarer mutations of exons 9 (10%) and 13 (2%) have also been subjects of study. Exon 9 mutations negatively predict the response to imatinib treatment and are largely specific to intestinal GISTs (14). Although they are rare, GISTs with mutations in exon 13 seem to have poor prognoses (27). For the PDGFR-α gene, approximately 80% of the mutations occur in exon 18 and are missense mutations (Asp842Val) that lead to imatinib resistance (4). Finally, there is a minority of GISTs that manifest no mutations (wild type). Wild-type GISTs generally have better prognoses than mutant GISTs. Typically, they affect pediatric patients and are often associated with congenital syndromes such as NF1 or Carney-Stratakis syndrome (16).
In our series, three out of five recurrences were KIT exon 11 deletions leading to Trp557_Lys558del at the protein level. Such a deletion has previously been linked to malignant behavior (28).
Gender may be an underestimated contributing factor of poor prognosis. In some studies, gender emerged as an independent predictor, even in multivariate analyses (9,10).
In our series, we did not have positive microscopic margins. The impact of microscopically negative margins remains controversial, and there is no evidence that extensive resections are related to a better survival rate. Some studies indicate that resection status influences the patient outcome (29), but this indication is not universally accepted. A study by DeMatteo et al. (9) involving over 200 cases analyzed the impact of margin status in patients with primary GISTs who underwent complete resections. Their conclusions indicated that the status of the microscopic margins of resection neither predicted recurrence nor affected survival. Tumor size was the most prognostic factor of survival. Thus, wide resections are not recommended, and because GISTs rarely metastasize to lymph nodes, lymphadenectomies during resection are generally unnecessary (30).
Laparoscopy was not associated with a significantly shorter hospitalization time. We performed two laparoscopies to open conversions. Tumor size and anatomical site are the most important factors to take into account when considering laparoscopy. The latest NCCN guidelines (31) assert that laparoscopy is reasonably safe and feasible for patients with low-risk gastric GISTs that are smaller than 5 cm. The proximity to the GE junction is a relatively frequent cause of open conversion, as reported in related literature (32). Although published data on laparoscopy for GISTs of the small intestine and other sites are sparse, clear recommendations for their laparoscopic resection are anticipated.
In conclusion, to date, there is no full agreement regarding the best GIST risk assessment. Many risk stratification scales have been published and tested, but none are exceedingly effective in predicting prognosis. Although tumor size, mitotic counts and anatomical location are the most well-established predictors, the reason for some unexpected results remains unclear, giving rise to GISTs being defined as tumors of “uncertain malignant potential”. The current knowledge of KIT and PDGFR-α mutations, their downstream effectors and alternative activating signaling pathways only partially explain such unpredictable behaviors. The reason for the unfavorable prognoses of small intestinal GISTs remains unknown. An accurate and standardized staging evaluation of the disease is mandatory to properly identify patients who could possibly take advantage of adjuvant therapy. On the basis of the present study and recent related evidence, we wish to emphasize two crucial points that were substantiated by our study. The first is that a tumor size of 7 cm should be considered the maximum threshold value for risk stratification. In 2002, Trupiano and coworkers (26) reported that tumors ≥7 cm in size were significantly more likely to metastasize than smaller tumors. More recently, through an evaluation of 2,537 GISTs from the Surveillance, Epidemiology and End Results (SEER) database, Woodal et al. (25) proposed a new clinical staging system with a tumor size cut-off measurement of 7 cm. The first tumor size cut-off value of the modified NIH consensus criteria suggested by Huang et al. (18) is 5 cm; similarly, Goh et al. (19) presented a proposal of modification of the Armed Forces Institute of Pathology (AFIP) criteria, putting together the very low and low groups of the original system. The second point concerns the level of mitosis. Mitotic counts have several limitations. The definition of a single HPF is not standardized. Depending on the observer, a count is not easily reproduced, and there may be significant intratumoral variability. It is also not clear which mitotic rate cut-off point should be applied. Some findings indicate that the single threshold value of five mitoses is not sufficient to distinguish the more from the less aggressive stromal tumors (18,25). Some studies (18,28) report that 10 mitoses per 50 HPFs significantly correlate with disease-specific survival. Singer et al. (33) found an even higher cut-off point of 15 mitoses per 50 HPFs as the strongest independent predictor. Furthermore, the Joensuu criteria (17), the NIH system revised by Huang (18) and the Goh-modified AFIP criteria (19) stratify the mitotic index into additional groups: <5, 6–10 and >10 mitoses per 50 HPFs.
Conclusions
Therefore, we feel that the current risk assessment criteria should be revised in light of the most recent evidence. Accordingly, a tumor size of 7 cm should be considered as the threshold value for malignancy, and smaller GISTs with low mitotic counts should be considered as tumors with a low-grade risk.
Non-randomized retrospective examination, consisting of a small sample size, are some limitations for this study. Nevertheless, our results agree with some previous larger studies as shown above. Surely further prospective studies will serve to confirm the research of our conclusions.
With the certainty that we will soon gain an encompassing knowledge of GISTs, we look forward to a single standardized staging system.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Neither the approval of the ethical committee nor the informed consent of patients were required by the type of research conducted (retrospective study).
References
- Sandvik OM, Søreide K, Kvaløy JT, et al. Epidemiology of gastrointestinal stromal tumours: Single-institution experience and clinical presentation over three decades. Cancer Epidemiol 2011;35:515-20. [Crossref] [PubMed]
- Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol 2002;33:459-65. [Crossref] [PubMed]
- Rubin JL, Sanon M, Taylor DC, et al. Epidemiology, survival, and costs of localized gastrointestinal stromal tumors. Int J Gen Med 2011;4:121-30. [PubMed]
- Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med 2006;130:1466-78. [PubMed]
- Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 1998;279:577-80. [Crossref] [PubMed]
- Heinrich MC, Corless CL, Demetri GD, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol 2003;21:4342-9. [Crossref] [PubMed]
- Joensuu H, Roberts PJ, Sarlomo-Rikala M, et al. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med 2001;344:1052-6. [Crossref] [PubMed]
- Benjamin RS, Blanke CD, Blay JY, et al. Management of gastrointestinal stromal tumors in the imatinib era: selected case studies. Oncologist 2006;11:9-20. [Crossref] [PubMed]
- DeMatteo RP, Lewis JJ, Leung D, et al. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg 2000;231:51-8. [Crossref] [PubMed]
- Rutkowski P, Nowecki ZI, Michej W, et al. Risk criteria and prognostic factors for predicting recurrences after resection of primary gastrointestinal stromal tumor. Ann Surg Oncol 2007;14:2018-27. [Crossref] [PubMed]
- Dematteo RP, Heinrich MC, El-Rifai WM, et al. Clinical management of gastrointestinal stromal tumors: before and after STI-571. Hum Pathol 2002;33:466-77. [Crossref] [PubMed]
- Dematteo RP, Ballman KV, Antonescu CR, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet 2009;373:1097-104. [Crossref] [PubMed]
- Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol 2005;29:52-68. [Crossref] [PubMed]
- Miettinen M, Makhlouf H, Sobin LH, et al. Gastrointestinal stromal tumors of the jejunum and ileum: a clinicopathologic, immunohistochemical, and molecular genetic study of 906 cases before imatinib with long-term follow-up. Am J Surg Pathol 2006;30:477-89. [Crossref] [PubMed]
- Dematteo RP, Gold JS, Saran L, et al. Tumor mitotic rate, size, and location independently predict recurrence after resection of primary gastrointestinal stromal tumor (GIST). Cancer 2008;112:608-15. [Crossref] [PubMed]
- Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol 2006;23:70-83. [Crossref] [PubMed]
- Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol 2008;39:1411-9. [Crossref] [PubMed]
- Huang HY, Li CF, Huang WW, et al. A modification of NIH consensus criteria to better distinguish the highly lethal subset of primary localized gastrointestinal stromal tumors: a subdivision of the original high-risk group on the basis of outcome. Surgery 2007;141:748-56. [Crossref] [PubMed]
- Goh BK, Chow PK, Yap WM, et al. Which is the optimal risk stratification system for surgically treated localized primary GIST? Comparison of three contemporary prognostic criteria in 171 tumors and a proposal for a modified Armed Forces Institute of Pathology risk criteria. Ann Surg Oncol 2008;15:2153-63. [Crossref] [PubMed]
- Gold JS, Gonen M, Gutierrez A, et al. Development and validation of a prognostic nomogram for recurrence-free survival after complete surgical resection of localised primary gastrointestinal stromal tumour: a retrospective analysis. Lancet Oncol 2009;10:1045-52. [Crossref] [PubMed]
- NCNN. NCNN Clinical Practice Guidelines in Oncology, Soft tissue sarcoma, 2009.
- Casali PG, Jost L, Reichardt P, et al. Gastrointestinal stromal tumours: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol 2009;20 Suppl 4:64-7. [PubMed]
- Jovine E, Biolchini F, Lerro FM, et al. Malignant gastrointestinal stromal neoplasm. Surgery 2006;140:832-4. [Crossref] [PubMed]
- Imatinib Mesylate or Observation Only in Treating Patients Who Have Undergone Surgery for Localized Gastrointestinal Stromal Tumor. Available online: https://clinicaltrials.gov/ct2/show/NCT00103168
- Woodall CE 3rd, Brock GN, Fan J, et al. An evaluation of 2537 gastrointestinal stromal tumors for a proposed clinical staging system. Arch Surg 2009;144:670-8. [Crossref] [PubMed]
- Trupiano JK, Stewart RE, Misick C, et al. Gastric stromal tumors: a clinicopathologic study of 77 cases with correlation of features with nonaggressive and aggressive clinical behaviors. Am J Surg Pathol 2002;26:705-14. [Crossref] [PubMed]
- Lasota J, Miettinen M. Clinical significance of oncogenic KIT and PDGFRA mutations in gastrointestinal stromal tumours. Histopathology 2008;53:245-66. [Crossref] [PubMed]
- Martín J, Poveda A, Llombart-Bosch A, et al. Deletions affecting codons 557-558 of the c-KIT gene indicate a poor prognosis in patients with completely resected gastrointestinal stromal tumors: a study by the Spanish Group for Sarcoma Research (GEIS). J Clin Oncol 2005;23:6190-8. [Crossref] [PubMed]
- Langer C, Gunawan B, Schuler P, et al. Prognostic factors influencing surgical management and outcome of gastrointestinal stromal tumours. Br J Surg 2003;90:332-9. [Crossref] [PubMed]
- Novitsky YW, Kercher KW, Sing RF, et al. Long-term outcomes of laparoscopic resection of gastric gastrointestinal stromal tumors. Ann Surg 2006;243:738-45; discussion 745-7. [Crossref] [PubMed]
- Demetri GD, von Mehren M, Antonescu CR, et al. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw 2010;8 Suppl 2:S1-41;quiz S42-4.
- Nguyen SQ, Divino CM, Wang JL, et al. Laparoscopic management of gastrointestinal stromal tumors. Surg Endosc 2006;20:713-6. [Crossref] [PubMed]
- Singer S, Rubin BP, Lux ML, et al. Prognostic value of KIT mutation type, mitotic activity, and histologic subtype in gastrointestinal stromal tumors. J Clin Oncol 2002;20:3898-905. [Crossref] [PubMed]