HER-2/neu gene amplification in gastric adenocarcinoma and its relationship with clinical and pathological findings
Introduction
Cancer, a disease with unchecked abnormal cells proliferation affecting adjacent tissues; is the second leading cause of death in human beings after heart diseases. Cancer cells can spread through the blood or lymph to other parts of the body (1).
Upper gastrointestinal cancers, including gastric cancer, are among the most common cancers (2-6). Reports from different parts of the world revealed very different results, but a higher incidence of gastric cancer in males compared to females is common (7).
A report from Iran [2012] showed that 11.4% of all cancer cases were attributable to gastric cancer, one which was considered as the second most prevalent cancer in the country. It was also presumed to be the most deadly cancer in Iran (GLOBOCAN, 2012), accounting for 15.5% of all mortalities caused by cancer (8).
Since gastric cancer is mainly diagnosed in advanced stages, identifying patients at risk using sensitive and specific diagnostic methods seems very useful. Despite the decline in gastric cancer rates in recent decades, it is still the major cause of mortality due to cancer. It has been shown that 39% of deaths due to cancer are related to gastric cancer (3).
Investigations showed that more than 750,000 people die annually from gastric cancer, and men are at risk for gastric cancer 2 times more than women. Gastric adenocarcinoma is the most common type of gastric cancer originating from the glandular tissues of the stomach. Other types of gastric cancer are lymphoma and sarcoma which originates in lymphoid and connective tissues, respectively (9,10).
Oncogenes come from certain genes called proto-oncogenes. Proto-oncogene products induce proliferation, but oncogenes are activated in elevated levels than normal. Proto-oncogene involved in cancer development can be divided into several groups, such as growth factors and cell-surface receptors like erythroblastosis oncogene B (erbB) or HER-2/neu. One of the most important abnormalities leading to cancer is oncogene over-expression.
Gene amplification means an increase in the number of copies in a limited area of the chromosome that leads to more than two copies of genes in that area. As a result, it causes an increase of critical genes involved in cancer onset and its progression (11). Of growth factors a cell needs, an epidermal growth factor (EGF) is the most famous one which has receptors on the surface of target cells called EGF receptor (EGFR) and HER-2 receptor (12).
HER-2/neu is the most important amplified oncogene in gastric cancer. Amplified HER-2/neu gene and its over-expression have been reported in patients with gastric cancer. HER-2/neu proto-oncogene, located on chromosome 17 (q12-21), is a transmembrane glycoprotein consisting of 125 amino acids and weighing185Kd. HER-2/neu codes an intrinsic tyrosine kinase activity, which is one of a four-member family of the EGF receptor. Normally, HER-2/neu is expressed in numbers of cells and tissues, and plays important roles in intracellular signaling, growth, differentiation, survival, and cell adhesion pathways (13).
Compared to the microscopic techniques, molecular techniques such as polymerase chain reaction (PCR) are faster, more accurate, cheaper, and easier (14). PCR is a semi-quantitative method to measure the amplification of HER-2/neu gene. Number of genes like TATA-box binding protein and INFγ (interferon gamma) is clear in cell structures, making them potent genes used as controls. In this method, the target gene with unknown copy number and the control gene with the known copy number (two copies per cell) are simultaneously amplified in a tube. Increased level of target gene is determined by the ratio of target gene and control band’s intensities in tumor samples in comparison with normal tissues, giving the relative copy number of the target gene (15).
As the northern and northwestern regions of Iran are high-risk areas for gastric cancer, compared to other geographical areas, this study was designed to measure the HER-2/neu gene expression in tissue samples of patients with gastric adenocarcinoma through PCR (after selecting INFγ gene as a control gene with two constant copies in cells).
Methods
In this case control study, 80 paraffin-embedded tissue samples of the gastric adenocarcinoma cases were selected from our academic hospital, pathology department [2006–2011]. This study was approved by Golestan University of Medical Sciences (Ethics Committee IR.GOUMS.REC.1395.290) and the informed consent was taken from all the patients.
Samples were taken from those cases that underwent gastrectomy (totally or partially). Demographic data such as age and gender were recorded and a pathologist reported the tumor size and staging of tumor.
Staging of tumor has been done for all cases based on data obtained during the main surgery recoding in the medical records and the pathological findings.
Ethical consideration
The study protocol has been approved in the local ethical committee of Golestan University of Medical Sciences. The informed consent has been taken during the main surgery for all becoming procedures.
Deparaffinization
A 6-micron section of paraffin blocks had been prepared and stored in sterile micro tubes. A deparaffinization method was used to extract DNA. For deparaffinization, we firstly added 1 mL xylol (Merck, Germany) to each vial and put them into thermo mixer devices (Eppendorf, Germany) in a temperature of 59 °C for 10 minutes; then, we centrifuged them for 10 min in 12,000 rpm. This step was taken again on the pellet of vial bottom. Finally, after supplying water with alcohol, the pellet without paraffin was used to extract DNA. For so doing, TBE (200 µL) and lysis buffer (400 µL) were added to the deparaffinized pellet and it was kept for one hour in a thermocycler. Then, we added chloroform (60 µL) to it and kept it for 3–5 minutes at room temperature. After 2 minutes of centrifugation, the supernatant was moved into another 1.5 mL microtome. Certain ratios of precipitation buffers and distilled water were added to it and it was placed at room temperature. After centrifugation, 100 µL of NaCl was added to the pellet, and after a one-minute vortex, 300 µL of ice ethanol was added to it and it froze at −20 °C for one day.
PCR procedure
Primer sequences used for HER-2 and INFγ (internal control) were:
F: 5'CATCAACTGCACCCACTCCT3' R: 5'GCAGCAGTCTCCGCATCGTG3' and F: 5'ATG AAA TAT ACA AGT TAT ATCTTG GCTTT3' R: 5'GAT GCT CTT CGA CCT CGA AAC AGC AT3', respectively. Both primer parameters, including primer dimers, and a lack of connection failure of the 3’ ends were determined by the Oligo 5 software. Primer pair products were over 100 and 150 bp for HER-2/neu and INFγ genes, respectively. PCR reactions were performed in a final volume of 25 µL. PCR mixture was comprised of 2.5 µL PCR buffer, 1.5 µL MgCl2, 10 pmol of each primer, 1 µL dNTPs, 0.5 µL Smar Taq DNA Pol, and 5 µL genomic DNA. Water was added to bring the mixture to the final volume of 25 µL. Thermo cycles (Eppendorf, Germany) and thermal conditions for PCR initiated denaturation at 94 °C for 10 minutes followed by 35 cycles including denaturation at 94 °C for 1 minute, annealing at 62 °C for 1 minute, extension at 70 °C for 1 minute, and the final expansion step was done at 72 °C for 10 minutes.
The PCR products were cast in the wells of 1.5% agarose gel. A 100-bp DNA Ladder (SM0313Fermentase) was poured in one of the wells. Since the good answer was obtained by PCR reaction, the same program was repeated with more samples, and the rest of the PCR samples run on 1.5% gel electrophoresis. The proportion of Band intensity or density of HER-2/neu to INFγ band’s for each sample was determined using the Image J software. The app was downloaded from http://rsbweb.nih.gov/ij. Considering the relative copy number of the HER-2/neu gene in each sample and gene amplification, a 2-fold or more increase in HER-2/neu band intensity over INFγ band intensity was observed.
Results
Tissue samples were taken from 80 gastric adenocarcinoma cases with mean (standard deviation) age of 67.92 (12.03) years. Mean tumor size was 2.96 (0.66) cm and 50 of them were males (62.5%). Eighteen patients (22.5%) were stage 3 and 62 patients (77.5%) were stage 4.
Amplification of HER-2/neu (Figure 1) showed overexpression of this gene in 58 samples (72.5%).
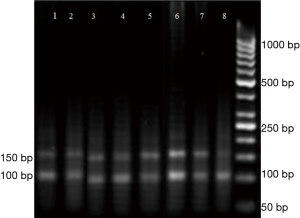
As shown in Table 1, HER-2/neu overexpression was detected in 82.3% of stage 4 tumors versus 38.9% of cases with stage 3 tumors. This difference was statistically significant (P value =0.000).
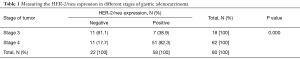
Full table
Tumor sizes analysis showed that the expression of HER-2/neu tumors and significantly larger than 3 cm in size can be seen (P value =0.000).
HER-2/neu overexpression was found in 40 males (80%) and 18 females (60%) (P value =0.052). There was no significant difference in HER-2/neu gene overexpression between cases younger and older than 60 years old (P value =0.098).
Discussion
Any increase in parts of genome containing oncogenes, such as HER-2/neu, would play a key role in the onset and development of tumors (16,17). Overexpression of HER-2/neu gene has been reported in 92–95% of gastric cancer cases (18,19). Routine methods used for assessing gene expression are immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH). Despite their simplicity, IHC method is easy, simple, fast, and low-cost that detects a 2-fold increase in gene amplification even in small amounts of tumor tissue. Techniques have low accuracy and often yield false-positive results in patients with IHC+ (20).
PCR In this study, HER-2/neu gene amplification in gastric adenocarcinoma samples using PCR method showed an increased level of HER-2/neu gene in 58 out of 80 samples.
Overexpression of HER-2/neu gene amplifications has been reported in more than 30% of breast, as well as colorectal cancer cases in other studies (19,21,22).
Furthermore, there is a direct relationship between HER-2/neu and gastric carcinoma stages which a study conducted by García et al. verified it (23). In a study by Al-Moundhri et al., the frequency of the her2 marker was obtained in 54% of patients with gastric cancer (16). Park et al. found that HER-2/neu is an important marker in the prognosis of the disease. They studied 82 patients with the gastric adenocarcinoma that 29 samples (15.9%) of them showed HER-2/neu marker and a lower 5-year survival rate was reported in HER-2/neu positive cases (24).
Overexpression of HER-2/neu gene may be the result of high gene transcription of HER-2/neu. Increased transcription of the gene may be directed by activating mutations in genes controlling HER-2/neu expression or increased expression of transcription factors involved in HER-2/neu transcription (25,26).
Conclusions
Present results showed that HER-2/neu gene expression is significantly more seen in stage 4 of gastric cancer with a larger size of mass. Older age and male sex were also associated with a higher level of HER-2/neu gene expression, although not significant.
Acknowledgements
This paper was extracted from MSc thesis dedicated to achieve MSc degree in Genetic.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by Golestan University of Medical Sciences (Ethics Committee IR.GOUMS.REC.1395.290) and the informed consent was taken from all the patients.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- DavoodAbadi A, Sharifi H, Erfan N, et al. An epidemiologic and clinical survey on gastric cancer patients refered to Shahid Beheshti Hospital of Kashan (1994-2001). Razi Journal of Medical Sciences 2003;10:211-20.
- Malekzadeh R, Derakhshan MH, Malekzadeh Z. Gastric cancer in Iran: epidemiology and risk factors. Arch Iran Med 2009;12:576-83. [PubMed]
- Babaei M, Pourfarzi F, Yazdanbod A, et al. Gastric cancer in Ardabil, Iran--a review and update on cancer registry data. Asian Pac J Cancer Prev 2010;11:595-9. [PubMed]
- Krejs GJ. Gastric cancer: epidemiology and risk factors. Dig Dis 2010;28:600-3. [Crossref] [PubMed]
- Androli T, Carpenter C, Griggs R, et al. Diseases of the liver and biliary system. Cecil's Essentials of Medicine 7th ed. USA: WB Saunders Company, 2007;23.
- Haidari M, Nikbakht MR, Pasdar Y, et al. Trend analysis of gastric cancer incidence in Iran and its six geographical areas during 2000-2005. Asian Pac J Cancer Prev. 2012;13:3335-41. [Crossref] [PubMed]
- Kavousi A, Bashiri Y, Mehrabi Y, et al. Identifying high-risk clusters of gastric cancer incidence in Iran, 2004 - 2009. Asian Pac J Cancer Prev 2014;15:10335-7. [Crossref] [PubMed]
- DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin 2014;64:252-71. [Crossref] [PubMed]
- Jarrell BE. Schwartz's principles of surgery. LWW; 2005.
- O'Hagan RC, Chang S, Maser RS, et al. Telomere dysfunction provokes regional amplification and deletion in cancer genomes. Cancer Cell 2002;2:149-55. [Crossref] [PubMed]
- Orangy E, Hojati Z, Ghaedi K, et al. Comparison of the HER2/neu gene amplification assesment by differential PCR and immunohistochemistry in breast cancer patients in Isfahan. Journal of Shahrekord University of Medical Sciences. 2012;13:71-82.
- Tapia C, Savic S, Wagner U, et al. HER2 gene status in primary breast cancers and matched distant metastases. Breast Cancer Res 2007;9:R31. [Crossref] [PubMed]
- Barberis M, Pellegrini C, Cannone M, et al. Quantitative PCR and HER2 testing in breast cancer: a technical and cost-effectiveness analysis. Am J Clin Pathol 2008;129:563-70. [Crossref] [PubMed]
- Zadrożny M, Smolarz B, Romanowicz-Makowska H, et al. Genetic analysis of HER-2/neu gene amplification in paraffin embedded tumour tissue in women with breast cancer. Pol J Pathol 2002;53:189-93. [PubMed]
- Al-Moundhri MS, Nirmala V, Al-Hadabi I, et al. The prognostic significance of p53, p27 kip1, p21 waf1, HER-2/neu, and Ki67 proteins expression in gastric cancer: a clinicopathological and immunohistochemical study of 121 Arab patients. J Surg Oncol 2005;91:243-52. [Crossref] [PubMed]
- Kuwahara Y, Tanabe C, Ikeuchi T, et al. Alternative mechanisms of gene amplification in human cancers. Genes Chromosomes Cancer 2004;41:125-32. [Crossref] [PubMed]
- Pauletti G, Godolphin W, Press MF, et al. Detection and quantitation of HER-2/neu gene amplification in human breast cancer archival material using fluorescence in situ hybridization. Oncogene 1996;13:63-72. [PubMed]
- Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 1989;244:707-12. [Crossref] [PubMed]
- Jacobs TW, Gown AM, Yaziji H, et al. HER-2/neu protein expression in breast cancer evaluated by immunohistochemistry. A study of interlaboratory agreement. Am J Clin Pathol 2000;113:251-8. [Crossref] [PubMed]
- Venter DJ, Tuzi NL, Kumar S, et al. Overexpression of the c-erbB-2 oncoprotein in human breast carcinomas: immunohistological assessment correlates with gene amplification. Lancet 1987;2:69-72. [Crossref] [PubMed]
- Ghaffarzadegan K, Sharifi N, Vosooghynia H, et al. HER2/neu expression in colon adenocarcinoma and its correlation with clinicopathologic variables. IJBMS 2006;1:64-9.
- García I, Vizoso F, Martín A, et al. Clinical significance of the epidermal growth factor receptor and HER2 receptor in resectable gastric cancer. Ann Surg Oncol 2003;10:234-41. [Crossref] [PubMed]
- Park DI, Yun JW, Park JH, et al. HER-2/neu amplification is an independent prognostic factor in gastric cancer. Dig Dis Sci 2006;51:1371-9. [Crossref] [PubMed]
- Hurst HC. Update on HER-2 as a target for cancer therapy: the ERBB2 promoter and its exploitation for cancer treatment. Breast Cancer Res 2001;3:395-8. [Crossref] [PubMed]
- Scott GK, Chang CH, Erny KM, et al. Ets regulation of the erbB2 promoter. Oncogene 2000;19:6490-502. [Crossref] [PubMed]


