The correlations between serum amphiregulin and other clinicopathological factors in colorectal cancer
Introduction
Amphiregulin (AREG) is an epidermal growth factor receptor (EGFR) ligand. It highly expressed in several cancers including CRC (1,2). AREG plays a pro-neoplastic effect through autocrine and paracrine pathways (3-5). Overexpression of AREG was demonstrated in more than fifty percent in both primary and liver metastatic tumors (1). Subsequent studies have shown high AREG expression of primary CRC tumors correlated with the poor clinicopathologic parameters including depth of tumor invasion, nerve invasion, liver metastasis and lower survival rate (2,6,7). Here we investigated the serum level of AREG in patients with CRC and its correlation with clinicopathological parameters.
Methods
Patients
Between August 2013 and March 2014, 120 consecutive pathologically confirmed CRC patients treated at the King Chulalongkorn Memorial Hospital (KCMH) were enrolled. Patients were at least 15 years of age and provided written informed consent. There was no other malignancy diagnosed in all patients. The study has been approved by the institutional review board of faculty of Medicine, Chulalongkorn University.
Level of serum AREG
Prior to any surgical treatment or systemic chemotherapy, 6 mL of heparinized blood was collected from subject. The samples were kept at room temperature for 30–60 minutes, then centrifuged at 1,600 ×g for 10 minutes. The serum was transferred to polypropylene-capped tube and kept frozen at −80 °C till further analysis. Serum AREG was measured using Human Amphiregulin DuoSet ELISA kit (R&D Systems, Minneapolis, MN, USA) followed the manufacturer instructions. All reactions were run duplicately. The range of detection for serum AREG is at 15.6–1,000 pg/mL.
Statistical analysis
All statistical analyses were performed using SPSS version 16.0 for Windows software (SPSS Inc., Chicago, IL, USA). The correlations between clinicopathological factors and serum AREG level were compared by the chi-squared test or t-test. P values <0.05 were considered statistically significant. The ROC method was used to identify the serum AREG cut off level in prediction of advanced diseases including stage IV and recurrent diseases.
Results
A total of 120 patients with CRC were enrolled into study between August 2013 and March 2014. The mean age was 64 years (range, 16–88 years). There were slightly more male than female. Most of patients had well to moderate histologic grade (77.5%) and pT1-3 (60.0%) tumor. Two-third of patients had stage I–III diseases. Other baseline characteristics were listed in Table 1.
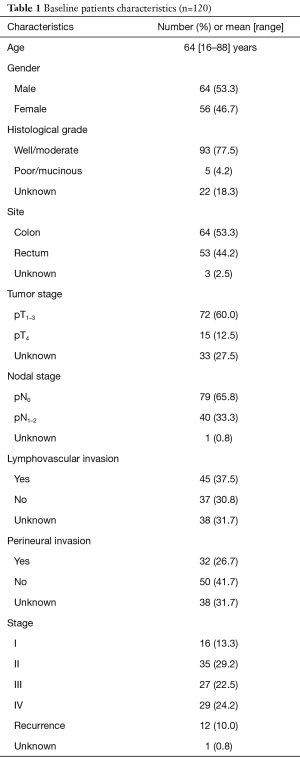
Full table
Serum amphiregulin and clinicopathological factors
The median and mean of serum AREG were shown in Table 2. The median of serum AREG in advanced diseases (metastatic or recurrent diseases) was significantly higher than localized disease 31.55 and 15.48 pg/mL, respectively (P=0.001).

Full table
To further explore the utility of serum AREG, we analyzed level of serum AREG by using ROC curve to identify a suitable cutoff value predicting advanced disease. Level of serum AREG at 25 pg/mL or more (high AREG), had the sensitivity and specificity of 51.2% and 76.9%, respectively, in prediction of advanced disease.
In high AREG group (n=39) there were significantly more patients with poorly differentiated/mucinous histological grade, distant metastasis, lymphovascular invasion, perineural invasion and stage IV/recurrent disease compared to low AREG group (n=81), P=0.014, P=0.001, P=0.016, P<0.001 and P=0.003, respectively (Table 3).
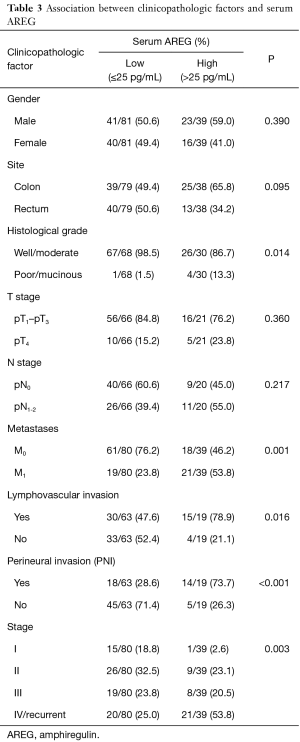
Full table
In metastatic disease, there were more patients with liver and/or peritoneal metastasis in high AREG group compared to low AREG group as shown in Table 4. All 15 patients with more than one metastatic site had high AREG (data not shown).
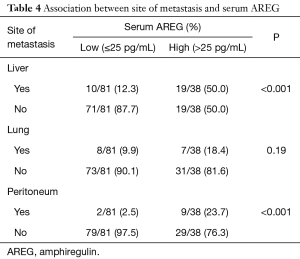
Full table
Discussion
In this cross-sectional study, the patients with advanced CRC had significantly higher serum AREG level than the patients with early CRC. The high serum AREG was associated with metastatic disease, higher TNM stage, poorly differentiated/mucinous histology, lymphovascular invasion and perineural invasion.
The serum AREG level was significantly higher in patients with advanced CRC compared to patients with early CRC. AREG is an EGFR binding ligand secreted by several cancer cells including CRC cells (4,5,8). Previously, the overexpression of AREG in primary colorectal cancer (CRC) associated with liver metastasis was shown in two retrospective studies (6,9). Kuramochi et al. also showed the correlation between the expression of AREG in liver metastatic tumor and primary CRC (10). In current study, patients with advanced CRC had significantly higher serum AREG levels. To our knowledge, this was the first to demonstrate serum AREG level associated with advanced disease. AREG was expressed and secreted by CRC cells either from primary or metastatic sites, the serum AREG level was potentially a marker correlated with CRC tumor burden.
High serum AREG was associated with other poor prognostic factors in CRC. In current study, it was associated with higher histologic grade, lymphovascular invasion, perineural invasion and higher stage. Previous studies of AREG in CRC depicted that AREG expression in primary tumor correlated with poor prognostic characteristics including tumor invasion, and nerve invasion (2,6,7) and some are predictive marker for liver metastasis (6,9). In 2010, Li et al. (7) demonstrated significant correlation between serum AREG level and vascular invasion. However, the authors did not provide the detail findings of serum AREG and the statistical analytical method was different to current study. Our findings supported high serum AREG as a poor prognostic factor in CRC.
Limitation with sample size and inclusion of all stages, we were not able to do survival analysis to confirm the independent prognostic value of serum AREG in CRC.
In summary, patients with advanced CRC had higher serum AREG level than patients with early CRC. High serum AREG (>25 pg/mL) was associated with advanced diseases and poor pathologic factors in CRC. It is potentially a prognostic marker in CRC.
Acknowledgements
Funding: This study was funded by the Ratchadapisek Sompoch Endowment Fund (2015), Chulalongkorn University (CU-58-001-HR).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the institutional review board of the Faculty of Medicine, Chulalongkorn University (300/56). The informed consents were obtained from all patients.
References
- Ciardiello F, Kim N, Saeki T, et al. Differential expression of epidermal growth factor-related proteins in human colorectal tumors. Proc Natl Acad Sci U S A 1991;88:7792-6. [Crossref] [PubMed]
- Ohchi T, Akagi Y, Kinugasa T, et al. Amphiregulin is a prognostic factor in colorectal cancer. Anticancer Res 2012;32:2315-21. [PubMed]
- Guzman MJ, Shao J, Sheng H. Pro-neoplastic effects of amphiregulin in colorectal carcinogenesis. J Gastrointest Cancer 2013;44:211-21. [Crossref] [PubMed]
- Culouscou JM, Remacle-Bonnet M, Carlton GW, et al. Colorectum cell-derived growth factor (CRDGF) is homologous to amphiregulin, a member of the epidermal growth factor family. Growth Factors 1992;7:195-205. [Crossref] [PubMed]
- Culouscou JM, Remacle-Bonnet M, Garrouste F, et al. Simultaneous production of IGF-I and EGF competing growth factors by HT-29 human colon cancer line. Int J Cancer 1987;40:646-52. [Crossref] [PubMed]
- Yamada M, Ichikawa Y, Yamagishi S, et al. Amphiregulin is a promising prognostic marker for liver metastases of colorectal cancer. Clin Cancer Res 2008;14:2351-6. [Crossref] [PubMed]
- Li XD, Miao SY, Wang GL, et al. Amphiregulin and epiregulin expression in colorectal carcinoma and the correlation with clinicopathological characteristics. Onkologie 2010;33:353-8. [Crossref] [PubMed]
- Pommier G, Culouscou JM, Garrouste F, et al. CRGF: an autocrine growth factor associated with colorectal carcinomas. Ann N Y Acad Sci 1988;551:382-4. [Crossref] [PubMed]
- Watanabe T, Kobunai T, Yamamoto Y, et al. Prediction of liver metastasis after colorectal cancer using reverse transcription-polymerase chain reaction analysis of 10 genes. Eur J Cancer 2010;46:2119-26. [Crossref] [PubMed]
- Kuramochi H, Nakajima G, Kaneko Y, et al. Amphiregulin and Epiregulin mRNA expression in primary colorectal cancer and corresponding liver metastases. BMC Cancer 2012;12:88. [Crossref] [PubMed]


