Appendiceal diverticulosis: a harbinger of underlying primary appendiceal adenocarcinoma?
Introduction
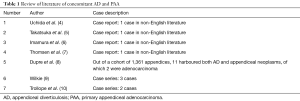
Primary appendiceal adenocarcinoma (PAA) is a rare entity, accounting for 0.5% of all gastrointestinal malignancies (1). While colonic diverticular is common, acquired appendiceal diverticulosis (AD) is rare, found in 0.004–2.1% of all appendectomies, and 0.2–0.66% of autopsy specimens (2,3). Their co-existence is extremely uncommon, with fewer than 10 case reports and case series worldwide (Table 1) (4-10). Some authors have postulated a correlation between the formation of AD and space-occupying appendiceal lesions that raise intra-luminal pressure (2,8,11,12). Is the presence of an appendiceal diverticulum a harbinger of an often silent but sinister appendiceal tumour?
Full table
Case presentation
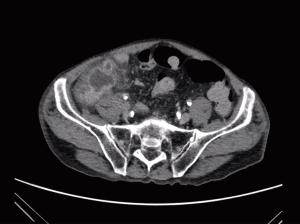
A middle-aged Chinese male presented to the emergency department of a tertiary hospital with five days duration of right iliac fossa (RIF) pain and anorexia. There was no fever, nausea or vomiting. Medical comorbidities include hypertension, dyslipidemia, peripheral vascular disease, chronic alcoholism and 30 pack years smoking history. No family history of malignancy was noted. Clinical examination revealed a localised tender RIF mass, and a normal digital rectal examination. Haemoglobin was 13.4 g/dL, leucocytosis 12.6×109/L with neutrophil left shift, and carcinoembryonic antigen was 3.5 µG/L. Contrasted abdominal computerised tomography (CT) scan (Figure 1) demonstrated a thickened appendix with 3 cm abscess at its base. There was no local or distant evidence of tumour. A diagnosis of acute perforated appendicitis was made, with a differential diagnosis of appendiceal diverticulitis.
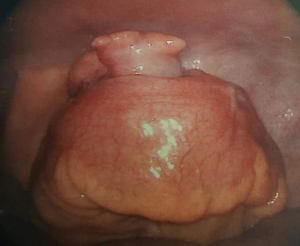
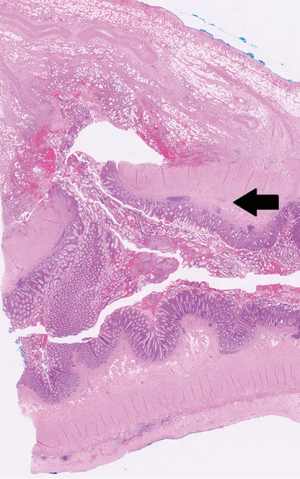
Emergency laparoscopic appendicectomy was performed. An appendiceal phlegmon was found densely adhered to the caecum and terminal ileum, without free perforation or intra-peritoneal disease. The appendiceal base was thickened and the caecal defect was closed with Vicryl stitches following appendicectomy (Figure 2). Histopathological examination showed an 8 cm long appendix, with a perforated acquired diverticulum near the appendiceal tip, associated with surrounding acute inflammation (Figure 3). There was an 8 mm well-differentiated PAA located 1 cm proximal to the tip, which was not perforated but had invaded the muscularis propria (Figure 4). Margins were clear and the base of the appendix was uninvolved by cancer. Final stage according to the American Joint Committee on Cancer 7th edition guidelines (13) was T2 Nx M0, with no lymph nodes harvested.
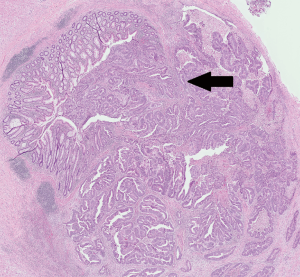
Post-operative recovery was unremarkable, and he was discharged 5 days later. He was advised for completion right hemicolectomy, but declined surgery. Colonoscopy revealed two sub-centimeter benign polyps. Interval CT scan 4 months later showed no metastatic disease or local recurrence.
Discussion
Early diagnosis of PAA is difficult, and most are discovered incidentally or present acutely with appendicitis (1,14-16). In this case, PAA was diagnosed due to a separate perforated AD. This may not be entirely fortuitous as recent literature (2,8,11,12) has suggested that the presence of an AD may herald an often silent appendiceal neoplasm. While most of these are benign, it becomes especially clinically pertinent when they harbour malignant pathologies.
AD is rare, occurring in 0.004–2.1% of appendectomies (2,3). ADs are false diverticula caused by mucosal herniation through the deficient muscularis propria. Raised intraluminal pressure may predispose to its formation, with luminal obstruction caused by benign inflammation, stricture, faecolith, polyps or mucin (3).
AD is associated with underlying appendiceal neoplasms in 7.1% to 48% of cases (8,11-13). Lamps et al. (11) reported a 42% association between low-grade appendiceal mucinous neoplasms and AAD, which was statistically significant compared to the respective incidences of these neoplasms and diverticula in the literature. Of 1,361 routine appendicectomy specimens, Dupre et al. (8) discovered 23 with AD, and 11 (48%) of these with concomitant appendiceal neoplasm, including carcinoids, mucinous adenomas, tubular adenomas and adenocarcinomas.
The relationship between AD and PAA is relatively unknown, and has been previously described in seven instances, of which four were in non-English journals (Table 1) (4-7), and two were described 50 to 100 years ago (Table 1) (9,10). PAA is also rare, accounting for 0.5% of all gastrointestinal malignancies, with an age-adjusted incidence of 0.12 cases per 1,000,000 people per year (17-19). Among appendiceal malignancies, 70% are carcinoids, 20% are cystadenocarcinomas and 10% are adenocarcinomas. Among adenocarcinomas, mucinous type (55%) is most common, often presenting with mucoceles or pseudomyxoma peritonei. This is followed by intestinal-type (34%) and adenocarcinoid type (11%). Each subtype varies in staging, management and prognosis.
The optimal management of intestinal-type PAA remains controversial, and guidelines are sparse. Right hemicolectomy is generally advocated as the preferred oncological intervention (17-24). Many studies demonstrate survival benefit of right hemicolectomies over appendectomies because the incidence of lymph node involvement is 66.7%. Right hemicolectomy led to upstaging of cancer in 38% of cases in Nitecki’s paper (1). This translates to a 73% 5-year survival rate in the right hemicolectomy group compared to 44% in the appendicectomy group, corresponding to the Surveillance, Epidemiology, and End Results database from 1973 to 2007, which showed a 55% 5-year survival (19). Smaller studies have suggested that appendicectomy may suffice for a highly-selected group of well-differentiated adenocarcinoma confined to the mucosa (23).
The role of chemotherapy is poorly understood. Extrapolating from colorectal cancer data, systemic with or without intraperitoneal chemotherapy may be used in the presence of nodal involvement. Controversy remains in those without nodal involvement, especially in perforated appendicitis or diverticulitis without gross malignant cell spillage. Might one presume that the cancer biology is similar to that of an obstructed colonic tumour with upstream colonic perforation? Nitecki (1) could not show a statistically-significant 5-year survival superiority when comparing perforated and non-perforated intestinal-type adenocarcinomas (39% versus 43%), postulating that there could be a decreased risk of peritoneal implantation in spilled appendiceal malignant cells compared its colonic counterpart.
Despite its rarity, recognising the association of AD and PAA has profound implications for surgeons and pathologists. In cases where malignancy is suspected pre-operatively or intra-operatively, and a grossly abnormal lesion is found, frozen section can be performed. We propose that a diagnosis of PAA of stage T2 and above is sufficient grounds (24) to proceed to a definitive right hemicolectomy in the same sitting, sparing the patient from repeated surgery.
As it may be difficult to differentiate acute inflammatory appendicitis and appendiceal neoplasms pre-operatively, surgeons should endeavour for atraumatic en-bloc appendectomies and avoid piece-meal removal. Some centres advocate dissecting the mesoappendix close to the appendiceal specimen, leaving the mesoappendix behind (25,26). We propose that surgeons should remove the entire mesoappendix to assess regional appendiceal lymphatic involvement (16).
Pathologists should recognise that up to half of AD may harbour a space-occupying lesion, which are easily missed if small: Dupre (8) described one case with concomitant AD and PAA which had a tiny focus of adenocarcinoma and was undiagnosed on initial routine representative sampling. Unlike its mucinous counterpart, intestinal-type adenocarcinomas rarely secrete mucin and are thus easily missed. Regular routine slicing involves longitudinal bisectioning at the base, middle and tip of the appendix, which may be insufficient (2). The presence of AD should prompt thorough, if not complete, histopathological examination of the entire appendectomy specimen.
To conclude, AD, though rare, is a harbinger of underlying appendiceal lesions that cause intraluminal hypertension. Both gastrointestinal surgeons and pathologists should be cognisant of its association with appendiceal neoplasms, especially malignant ones like PAA.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and its accompanying images.
References
- Nitecki SS, Wolff BG, Schlinkert R, et al. The natural history of surgically treated primary adenocarcinoma of the appendix. Ann Surg 1994;219:51-7. [Crossref] [PubMed]
- Deng YW, Yang HB, Feng KC, et al. Appendiceal diverticular disease. Formosan Journal of Surgery 2013;46:4-9. [Crossref]
- Abdullgaffar B. Diverticulosis and diverticulitis of the appendix. Int J Surg Pathol 2009;17:231-7. [Crossref] [PubMed]
- Uchida T, Hirano Y, Yoshida S, et al. A case of primary early appendiceal carcinoma detected by perforation of appendiceal diverticulum. Journal of Japan Surgical Association 2012;73:1144-8. [Crossref]
- Takatsuka S, Yamamoto A, Takagaki K. A Case of Appendiceal Carcinoma with Perforated Appendiceal Diverticulum. The Japanese Journal of Gastroenterological Surgery 2000;33:1710-3. [Crossref]
- Imamura H, Kawashita Y, Koga N, et al. Primary Adenocarcinoma Concomitant with Perforated Diverticulum of the Appendix - Report of a Case. Journal of Japan Surgical Association 2014;75:484-8. [Crossref]
- Thomsen JB, al-Suliman N, Kåg L, et al. Ugeskr Laeger 2000;162:3051-2. [Primary adenocarcinoma in appendiceal diverticulitis]. [PubMed]
- Dupre MP, Jadavji I, Matshes E, et al. Diverticular disease of the vermiform appendix: a diagnostic clue to underlying appendiceal neoplasm. Hum Pathol 2008;39:1823-6. [Crossref] [PubMed]
- Wilkie DP. Carcinoma of the appendix causing diverticula of the appendix and acute appendicular obstruction. Br J Surg 1920;8:392-6. [Crossref]
- Trollope MI, Lindenauer SM. Diverticulosis of the appendix: a collective review. Dis Colon Rectum 1974;17:200. [Crossref] [PubMed]
- Lamps LW, Gray GF Jr, Dilday BR, et al. The coexistence of low-grade mucinous neoplasms of the appendix and appendiceal diverticula: a possible role in the pathogenesis of pseudomyxoma peritonei. Mod Pathol 2000;13:495-501. [Crossref] [PubMed]
- Kallenbach K, Hjorth SV, Engel U, et al. Significance of acquired diverticular disease of the vermiform appendix: a marker of regional neoplasms? J Clin Pathol 2012;65:638-42. [Crossref] [PubMed]
- Edge S, Byrd DR, Compton CC, et al. editors. AJCC cancer staging Handbook. 7th edition. New York, NY: Springer-Verlag, 2010.
- González-Moreno S, Brun E, Sugarbaker PH. Lymph node metastasis in epithelial malignancies of the appendix with peritoneal dissemination does not reduce survival in patients treated by cytoreductive surgery and perioperative intraperitoneal chemotherapy. Ann Surg Oncol 2005;12:72-80. [Crossref] [PubMed]
- Ruoff C, Hanna L, Zhi W, et al. Cancers of the appendix: review of the literatures. ISRN Oncol 2011;2011:728579. [PubMed]
- Sugarbaker PH. Adenocarcinoma as a cause of acute appendicitis. Ann Emerg Surg 2017;2:1006.
- Kelly KJ. Management of Appendix Cancer. Clin Colon Rectal Surg 2015;28:247-55. [Crossref] [PubMed]
- O’Donnell ME, Badger SA, Beattie GC, et al. Malignant neoplasms of the appendix. Int J Colorectal Dis 2007;22:1239-48. [Crossref] [PubMed]
- McCusker ME, Coté TR, Clegg LX, et al. Primary malignant neoplasms of the appendix: a population-based study from the surveillance, epidemiology and end-results program, 1973-1998. Cancer 2002;94:3307-12. [Crossref] [PubMed]
- Turaga KK, Pappas SG, Gamblin T. Importance of histologic subtype in the staging of appendiceal tumors. Ann Surg Oncol 2012;19:1379-85. [Crossref] [PubMed]
- Englehardt RK, Durrani NK, Mittal VK. Management and outcomes in primary tumours of the appendix. J Cancer Ther 2010;1:174-80. [Crossref]
- Ito H, Osteen RT, Bleday R, et al. Appendiceal adenocarcinoma: long-term outcomes after surgical therapy. Dis Colon Rectum 2004;47:474-80. [Crossref] [PubMed]
- Hata K, Tanaka N, Nomura Y, et al. Early appendiceal adenocarcinoma. A review of the literature with special reference to optimal surgical procedures. J Gastroenterol 2002;37:210-4. [Crossref] [PubMed]
- Guraya SY, Almaramhy HH. Clinicopathological features and the outcome of surgical management for adenocarcinoma of the appendix. World J Gastrointest Surg 2011;3:7-12. [Crossref] [PubMed]
- Hsieh CS, Chen YL, Lee MH, et al. A lower costly laparoscopic appendectomy: our experience of more than 2000 cases. Int J Surg 2010;8:140-3. [Crossref] [PubMed]
- Domene CE, Volpe P, Heitor FA. Three port laparoscopic appendectomy technique with low cost and aesthetic advantage. Arq Bras Cir Dig 2014;27 Suppl 1:73-6. [Crossref] [PubMed]