Outcomes and complications of radiation therapy in patients with familial adenomatous polyposis
Introduction
Familial adenomatous polyposis (FAP) is an inherited autosomal dominant syndrome caused by mutations in the adenomatous polyposis coli (APC) gene resulting in an increased risk of multiple malignancies (1,2). The APC gene is a tumor suppressor that is an important regulator of transcription. While a majority of cases have a known family history of FAP, approximately 20% of APC mutations occur sporadically (1,2). Although FAP is most commonly associated with colorectal cancer, there are phenotypic variants of FAP that are associated with tumors throughout the body, such as Turcot syndrome which presents with CNS malignancies such as gliomas and medulloblastomas and Gardner syndrome with osteomas and desmoids (3). Colorectal cancer occurs in the majority of FAP patients, with a 90% lifetime risk of developing colorectal cancer and a median age of diagnosis of 35 (2).
Radiation therapy (RT) is an important treatment modality for malignancies affecting FAP patients, including colorectal cancer, desmoid tumors, medulloblastomas, and gliomas. Unfortunately, there are no data that describe the toxicity or risk of secondary malignancies among patients with FAP receiving RT. Our purpose was to analyze these clinical outcomes of patients with FAP treated with RT.
Methods
The Hereditary Gastrointestinal Cancer Registry (HGCR) is a resource for individuals with familial gastrointestinal cancers and enrolls individuals participating in research and/or clinically managed at a referral center for genetics and cancer research. All aspects of this study are approved by the Institutional Review Board. HGCR participants with a diagnosis of FAP were matched with records held in an institutional Electronic Data Warehouse (EDW). Our query of the EDW was limited to patients with FAP who received RT as a part of their definitive or palliative treatment. Patients with incomplete staging, treatment, or long-term follow-up were excluded from analysis.
A total of 36 patients were identified from the initial query of the terms “FAP” and “radiation”, “radiotherapy”, “RT”, or “brachytherapy” in the medical record. Basic demographic and clinical information were abstracted from the record, including age at diagnosis, gender, and type of tumor. Details including RT modality, dose, concurrent chemotherapy use and type, toxicity, recurrence, and development of secondary malignancies were collected and analyzed. Toxicity was defined by adverse events in organ systems within the RT treatment field and was graded by Common Terminology Criteria for Adverse Events version 4 (CTCAE v4) criteria. Acute toxicity was defined as an adverse event occurring during or within three months of delivery of RT. Adverse events occurring after three months of delivery of RT were defined as late toxicity. A secondary malignancy was defined as a new primary tumor developing in the area of the previous RT treatment field or a new leukemia. Local recurrence was defined as regrowth of a tumor at a previously treated site that was of the same pathology as the original tumor. Regional recurrence was defined as the tumor extending outside the boundary of the original tumor but not disseminated distantly.
Results
Of the 36 patients initially queried, a total of 18 were found to have both a genetic diagnosis of FAP and treatment that included RT. Out of those, detailed records of RT treatment were obtainable in 15 patients undergoing 18 treatment courses. In this population of 15 patients, eight were female, and seven were male. Radiotherapy treatment dates ranged from 1997 to 2014. The median age at first treatment with RT was 47 years old. Five patients had desmoid tumors, three had rectal cancer, two had prostate cancer, and one each of breast cancer, melanoma, medulloblastoma, gastric cancer, and a glioma of undetermined type. Eight patients received concurrent systemic therapy. Radiotherapy treatment intent was neo-adjuvant in two, definitive in three, adjuvant in seven, and palliative in six treatment courses. Except one patient treated with low dose rate brachytherapy for prostate cancer, all patients were treated with external beam RT. Eight patients had concurrent systemic therapies with RT (Table 1).
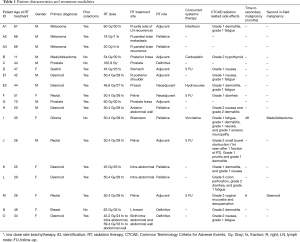
Full table
For the entire cohort, side effects were most often dermatitis, fatigue, nausea, and diarrhea. Overall, the highest CTCAE v4 toxicities were grade 1 in seven treatment courses (39%), grade 2 in five treatment courses (28%), and grade 3 in two treatment courses (11%). The most common toxicity was dermatitis, seen in 39% of treatment courses (Table 1).
For abdominal or pelvic RT patients, the rate of grade 3 and above acute toxicity was 20% (2/10 treatment courses in nine patients). The rate of grade 3 and above acute gastrointestinal toxicity was 10% (1/10 treatments in nine patients), even though all nine patients treated with abdominal or pelvic RT had previously undergone prophylactic proctocolectomy. This occurred in patient L who experienced grade 3 colonic microperforations 1 month after concluding RT for large recurrent abdominal desmoids. In general, GI toxicities in patients treated with abdominal or pelvic RT included diarrhea and nausea. One patient treated with adjuvant RT for rectal cancer experienced small bowel obstruction after one fraction of radiation therapy. Another treated with adjuvant RT to the pelvis and inguinal lymph nodes for low-lying rectal cancer experienced grade 3 vaginal mucositis. One patient received two courses of RT for the same intra-abdominal desmoid tumor. In this patient, re-irradiation was associated with only grade 1 dermatitis and fatigue.
Three patients were treated for rectal adenocarcinomas, all to 50.4 Gray (Gy). Patient F had received a near total colectomy with ileorectal anastomosis (IRA) in 1971. Her rectal cancer was found in 2002 and was located at 6 centimeters (cm) from the anal verge. She was treated with chemoradiation therapy with continuous infusion of fluorouracil (5 FU) followed by an abdominal peritoneal resection (APR). Patient J also received a subtotal colectomy and one year later was found to have a rectal adenocarcinoma. He received an APR followed by chemoradiation therapy. Neither of these patients had received a total prophylactic resection of the rectum. Patient M was found to have four adenocarcinomas at the time of proctocolectomy following an initial diagnosis of FAP. One of these was in the rectum and was associated with two positive lymph nodes. The patient received adjuvant RT to the pelvis following the initial proctocolectomy. No rectal cancer patients recurred locally. Patient F was found to have metastases 12 months after completion of the treatment of the primary tumor.
Five patients were treated for desmoid tumors with doses ranging from 43.2 to 59.2 Gy in 1.8 Gy fractions (median 50.4 Gy). Intra-abdominal tumors were treated to a lower dose (43.2 to 50.4 Gy, median 45 Gy) than abdominal wall/musculoskeletal desmoids (48.6 to 59.4 Gy, median 50.4 Gy) due to concern for radiation toxicity of visceral organs. Included were two large intra-abdominal tumors, one of which recurred locally (patient L). A third patient (patient O) was treated simultaneously for both abdominal wall and intra-abdominal desmoids with no reduction in tumor size but no growth at 3 months follow-up. One patient (patient E) was treated to a posterior shoulder desmoid after 98% debulking. Unfortunately, this tumor recurred 6 months later. The patient was re-treated to this area to 48.6 Gy followed by debulking surgery. There were no undue adverse events at retreatment. Two of five patients with desmoid tumors recurred locally within 5 years.
Of four courses of RT to the brain, late grade 2 hypothyroidism was seen in one patient treated at age 11 for medulloblastoma (patient B). Another patient treated for brainstem glioma experienced grade 1 sensory neuropathy, grade 1 nausea, grade 1 fatigue, and grade 1 dermatitis (patient I). Two courses of brain stereotactic radiosurgery (SRS) for melanoma metastases had no toxicity.
Second in-field malignancies occurred in two patients: one diagnosed with medulloblastoma 4 years after RT for a glioma, another with an abdominal desmoid eight years after RT for colorectal cancer (Table 1).
Discussion
This study represents the only known series evaluating toxicity, and secondary malignancy among patients with FAP treated with RT. It is also the largest RT study that we were able to identify for FAP-related desmoid tumors or colorectal cancer. In our cohort, we found that RT was well tolerated and associated with two cases of grade 3 toxicity and no grade 4 or higher toxicities observed. In patients who received pelvic or abdominal irradiation, there was a 20% risk of grade 3 acute toxicity. Overall the majority of the toxicities seen were acute with one instance of late grade 2 hypothyroidism observed. Two patients experienced second in-field malignancies, both of which were FAP-associated cancers. It is not known if RT increased the risk of secondary malignancy in these patients.
FAP is not known to be associated with an increase in RT toxicity and can be considered separately from the multiple established genetic syndromes that increase radiosensitivity, which leads to increased toxicity given the same dose of radiation. Known radiosensitive syndromes include ataxia telangiectasia, Fanconi’s anemia, basal cell nevus syndrome, and Li-Fraumeni (4-7).
Very little data exists on outcomes after adjuvant or neoadjuvant RT for rectal cancer after subtotal proctocolectomy. The few reports that mention the use of RT in the literature do not report on the details or side effects (8,9). The cumulative risk of rectal cancer after colectomy in FAP patients has been estimated at 4%, 5.6%, 7.9% and 25% at 5, 10, 15 and 20 years, respectively (10). The 5-year survival rate of patients with FAP diagnosed with rectal cancer after colectomy is reported to be 71% (11). While colorectal cancer is the cause of death in up to 48% of patients dying with a known diagnosis of FAP, those who undergo prophylactic colectomy are more likely to die of desmoid tumors, and periampullary malignancies (12). Excision of the entire colorectal mucosa is the treatment of choice to avoid later rectal cancers (13); however, the timing of completion proctectomy has been a matter of debate (14). The risk of cancer in the retained rectum increases sharply from 10% for patients under 50 to 30% after the age of 60 years (15,16). When rectal cancer is diagnosed in the retained rectal pouch, APR is usually the surgical treatment of choice (17-19). An ileal pouch-anal anastomosis is sometimes possible, but complications from adjuvant treatment such as enteritis or pouch failure can be increased. The risk of rectal cancers persists as long as any rectal mucosa remains.
Little is known regarding the risk of secondary cancers in patients with FAP resulting from RT treatments. The two in-field malignancies that occurred in our cohort of patients were medulloblastoma and desmoid, both of which are well described as malignancies associated with FAP (1-3). We identified one case report regarding two in-field malignancies after RT in a pediatric FAP patient not associated with typical FAP related tumors, but no other reports in the literature of similar cases (20).
Desmoid tumors were the most common FAP-associated tumor treated with RT in our study, comprising 33% of the patients. Desmoid tumors are non-malignant fibromatous proliferations and represent the second most common tumor in FAP patients overall, affecting 10–15% (21). They are the second most common cause of death in FAP patients after colorectal cancer (22), becoming fatal by local extension and compression of nearby vital structures. Desmoids associated with FAP are more likely to involve the abdomen than non-FAP desmoids. Larger retrospective reviews of the management of desmoid disease in patients with FAP have shown that most desmoids develop following a colectomy after a mean time of about 5 years (23). Surgery can be effective for bowel obstruction, which is the most common presenting symptom for intra-abdominal desmoids, but resection is also associated with a high morbidity and should not be attempted electively for intra-abdominal disease. Management often starts with systemic agents including nonsteroidal anti-inflammatory drugs (NSAIDS) and/or selective estrogen receptor blockers such as tamoxifen (23). If symptoms such as abdominal pain, nausea, vomiting, or diarrhea, are refractory to these agents, chemotherapy, RT and/or surgery are considered.
A published meta-analysis and review of the literature on desmoid tumors comparing RT to surgery for non-FAP patients showed that RT alone or in combination with surgery was most effective (24). In this meta-analysis, local control using RT alone was found to be 78% with a follow-up of 2.0 to 10.4 years between studies. However, of the five desmoid tumors in our study with follow-up data, two recurred locally. Similarly, the Rare Cancer Network analyzed 110 patients with desmoids and found that postoperative RT improved progression-free survival compared to surgery alone (25).
Because of variation in tumor location, size, and treatment intent in this small population, we are unable to determine whether FAP-related desmoids are at an increased risk of local recurrence compared to non-FAP-related desmoid tumors. The few prior reports of RT for desmoid tumors in patients with FAP show mixed results. Clark and colleagues reported no further growth after RT to one FAP-associated abdominal wall desmoid (26). An analysis from the Cleveland Clinic of three patients with FAP treated with RT showed no response to RT (27). Lastly, response to any therapy is difficult to measure as some show spontaneous regression in the absence of treatment.
Parsing the difference between local recurrence and new primary cancer can be difficult in patients with a strong predisposition to malignant transformation. While there were no local recurrences in our cohort of rectal cancer patients, FAP may predispose patients to a higher risk of second primary cancers due to polyposis. In the non-FAP population, rectal cancer patients treated with neoadjuvant or adjuvant RT experience local control rates between 5–16%. In-field second malignancies occurred in 2 of 18 treatments. It is difficult to draw conclusions regarding the etiology of these second primary cancers as patients with FAP inherently have a high risk of developing multiple primary cancers not associated with RT. To our knowledge, this is the largest published report of patients with FAP receiving RT. However, our study has limitations due to its small sample size. Multivariate analysis to control for cofactors was not feasible. It also shares the weaknesses of retrospective analysis including lack of standardized treatment and the potential for incomplete chart information.
Conclusions
In conclusion, RT was well tolerated with adverse effects consistent with non-FAP patients. While treating patients with FAP requires special attention to the risk of possible future malignancies, our data does not support foregoing standard of care RT. We do not believe this data represents an increased risk of secondary malignancy for FAP patients, as all second in-field cancers were diseases known to be associated with FAP itself. Desmoid tumors in patients with FAP tended to exhibit aggressive growth, but local control was achieved in some patients.
Acknowledgements
The authors thank Michelle Denney for the administrative support.
Funding: This research was supported in part by funding from the National Cancer Institute PO1-CA073992 and the Huntsman Cancer Institute.
Footnote
Conflicts of Interest: NJ Samadder received an honorarium for speaking from Cook Medical Inc., outside the submitted work; S Lloyd received an honorarium for panel participation from Sirtex. The other authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the institutional ethics board of University of Utah (No. 00030546) and written informed consent was obtained from all patients.
References
- Kennedy RD, Potter DD, Moir CR, et al. The natural history of familial adenomatous polyposis syndrome: a 24 year review of a single center experience in screening, diagnosis, and outcomes. J Pediatr Surg 2014;49:82-6. [Crossref] [PubMed]
- Lal G, Gallinger S. Familial adenomatous polyposis. Semin Surg Oncol 2000;18:314-23. [Crossref] [PubMed]
- Chowdhary UM, Boehme DH, Al-Jishi M. Turcot syndrome (glioma polyposis). Case report. J Neurosurg 1985;63:804-7. [Crossref] [PubMed]
- Pollard JM, Gatti RA. Clinical radiation sensitivity with DNA repair disorders: an overview. Int J Radiat Oncol Biol Phys 2009;74:1323-31. [Crossref] [PubMed]
- Morgan JL, Holcomb TM, Morrissey RW. Radiation reaction in ataxia telangiectasia. Am J Dis Child 1968;116:557-8. [PubMed]
- Zhang L, Yang M, Bi N, et al. ATM polymorphisms are associated with risk of radiation-induced pneumonitis. Int J Radiat Oncol Biol Phys 2010;77:1360-8. [Crossref] [PubMed]
- Andreassen CN, Alsner J. Genetic variants and normal tissue toxicity after radiotherapy: a systematic review. Radiother Oncol 2009;92:299-309. [Crossref] [PubMed]
- Ooi BS, Remzi FH, Gramlich T, et al. Anal transitional zone cancer after restorative proctocolectomy and ileoanal anastomosis in familial adenomatous polyposis: report of two cases. Dis Colon Rectum 2003;46:1418-23; discussion 1422-3. [Crossref] [PubMed]
- Vrouenraets BC, Van Duijvendijk P, Bemelman WA, et al. Adenocarcinoma in the anal canal after ileal pouch-anal anastomosis for familial adenomatous polyposis using a double-stapled technique: report of two cases. Dis Colon Rectum 2004;47:530-4. [Crossref] [PubMed]
- Heiskanen I, Järvinen HJ. Fate of the rectal stump after colectomy and ileorectal anastomosis for familial adenomatous polyposis. Int J Colorectal Dis 1997;12:9-13. [Crossref] [PubMed]
- De Cosse JJ, Bülow S, Neale K, et al. Rectal cancer risk in patients treated for familial adenomatous polyposis. The Leeds Castle Polyposis Group. Br J Surg 1992;79:1372-5. [Crossref] [PubMed]
- Arvanitis ML, Jagelman DG, Fazio VW, et al. Mortality in patients with familial adenomatous polyposis. Dis Colon Rectum 1990;33:639-42. [Crossref] [PubMed]
- Jang YS, Steinhagen RM, Heimann TM. Colorectal cancer in familial adenomatous polyposis. Dis Colon Rectum 1997;40:312-6. [Crossref] [PubMed]
- Smith JC, Schäffer MW, Ballard BR, et al. Adenocarcinomas After Prophylactic Surgery For Familial Adenomatous Polyposis. J Cancer Ther 2013;4:260-70. [Crossref] [PubMed]
- Bülow C, Vasen H, Järvinen H, et al. Ileorectal anastomosis is appropriate for a subset of patients with familial adenomatous polyposis. Gastroenterology 2000;119:1454-60. [Crossref] [PubMed]
- Nugent KP, Phillips RK. Rectal cancer risk in older patients with familial adenomatous polyposis and an ileorectal anastomosis: a cause for concern. Br J Surg 1992;79:1204-6. [Crossref] [PubMed]
- Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol 2012;30:1926-33. [Crossref] [PubMed]
- Ngan SY, Burmeister B, Fisher RJ, et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01.04. J Clin Oncol 2012;30:3827-33. [Crossref] [PubMed]
- Gérard JP, Conroy T, Bonnetain F, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol 2006;24:4620-5. [Crossref] [PubMed]
- Williams PA, Bhaijee F, Rezeanu L, et al. Two Metachronous Neoplasms in the Radiotherapy Fields of a Young Man With Familial Adenomatous Polyposis. J Investig Med High Impact Case Rep 2013;1:2324709613484302. [Crossref] [PubMed]
- Vasen HF, Möslein G, Alonso A, et al. Guidelines for the clinical management of familial adenomatous polyposis (FAP). Gut 2008;57:704-13. [Crossref] [PubMed]
- Half E, Bercovich D, Rozen P. Familial adenomatous polyposis. Orphanet J Rare Dis 2009;4:22. [Crossref] [PubMed]
- Soravia C, Berk T, McLeod RS, et al. Desmoid disease in patients with familial adenomatous polyposis. Dis Colon Rectum 2000;43:363-9. [Crossref] [PubMed]
- Nuyttens JJ, Rust PF, Thomas CR Jr, et al. Surgery versus radiation therapy for patients with aggressive fibromatosis or desmoid tumors: A comparative review of 22 articles. Cancer 2000;88:1517-23. [Crossref] [PubMed]
- Baumert BG, Spahr MO, Von Hochstetter A, et al. The impact of radiotherapy in the treatment of desmoid tumours. An international survey of 110 patients. A study of the Rare Cancer Network. Radiat Oncol 2007;2:12. [Crossref] [PubMed]
- Clark SK, Phillips RK. Desmoids in familial adenomatous polyposis. Br J Surg 1996;83:1494-504. [Crossref] [PubMed]
- Tsukada K, Church JM, Jagelman DG, et al. Systemic cytotoxic chemotherapy and radiation therapy for desmoid in familial adenomatous polyposis. Dis Colon Rectum 1991;34:1090-2. [Crossref] [PubMed]


