Overall and disease-free survival in patients treated with CRS + HIPEC with cisplatin and paclitaxel for gastric cancer with peritoneal carcinomatosis
Introduction
Advanced gastric cancer (GC) is an increasingly common finding and has generally been associated with a grim prognosis. The median survival for patients with peritoneal carcinomatosis (PC) caused by GC is only between 3 to 7 months, while the median survival increases up to 9.5−12 months in patients with PC, caused by GC, who underwent chemotherapy (1). The management of locally advanced GC (perioperative chemotherapy followed by gastrectomy and D2 lymphadenectomy) is based on solid clinical evidence (2). On the contrary the diagnosis of PC in addition to any stage of GC has been traditionally considered a cut off point for surgical intervention and has always been treated with palliative chemotherapy. This creates a situation where locally advanced cancers with positive lymph nodes are subject to aggressive multimodality treatment, whereas less advanced tumors with PC, which can be considered a result of loco regional extension of the primary cancer into the peritoneal cavity, are treated with palliative chemotherapy (3). Without any doubt PC caused by GC represents a poor long-term survival clinical situation. However, advanced GC with advanced lymphatic spread has a comparable prognosis. The difference between these two above mentioned scenarios is that PC can be diagnosed early with a staging laparoscopy, while lymphatic spread is usually a postoperative pathological diagnosis (3). Moreover these patients more frequently die for peritoneal spread of the disease than for distant metastases (77% of patients without distant metastasis die due to peritoneal progression of disease) despite a radical surgery and radio-chemotherapy regimes (4). In view of this and considering that the estimate of synchronous peritoneal metastases discovered at the time of gastrectomy is 20% (5), it is important to develop strategies to improve the prevention, diagnosis and treatment of PC caused by primary GC.
Even more serious problem is metachronous PC of recurrent GC. This has an incidence of 60% and it is a major cause of intestinal obstruction, fistula formation and bowel perforation (5). Sugarbaker hypothesized that the mechanism that can explain the high incidence of local recurrence and peritoneal metastases in gastrointestinal cancer is the “tumor cell entrapment”: manipulation of the cancer specimen during surgery results in free cancer cells in peritoneal space that can contaminate transected lymphatic channels, implant and result in local-regional recurrence diagnosed during follow-up. Even the most perfect primary cancer resection cannot prevent this contamination and systemic chemotherapy can poorly control this phenomenon because of the “plasma-peritoneal barrier”. Therefore hyperthermic intraperitoneal chemotherapy (HIPEC) could be considered to supplement meticulous surgical technique in selected patients (4).
The current combined treatment for peritoneal metastases (cytoreductive surgery (CRS) plus HIPEC) can be applied at three different moments in the natural history of a gastrointestinal cancer: (I) to prevent the peritoneal metastases in selected high risk patients with GC without PC (5); (II) to manage synchronous PC of primary GC (5); (III) to manage a metachronous PC from recurrent GC (5). The aim of the study is to analyze outcomes in terms of OS and DFS in patients with SPC and MPC from GC in our experience.
Methods
An analysis of prospectively collected data was conducted regarding patients who underwent CRS and HIPEC for advanced GC with peritoneal carcinomatosis. All patients with GC with PC treated with combined CRS and HIPEC from July 2011 to July 2016 at ASST Papa Giovanni XXIII (Bergamo, Italy) were considered. Patients were divided into two groups, those having synchronous peritoneal carcinomatosis (SPC) and those having metachronous peritoneal carcinomatosis (MPC). The main outcomes analyzed were overall survival (OS) and disease-free survival (DFS).
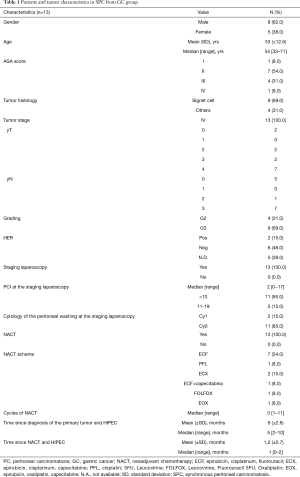
Tumor and patient characteristics analyzed in each group were gender, age, ASA score, tumor histology, tumor stage and grading, molecular classification based on HER2 status. In SPC group also other factors were analyzed, such as: the use of staging laparoscopy, the Peritoneal Cancer Index (PCI) and the cytology of the peritoneal washing at the staging laparoscopy, the use of neo-adjuvant chemotherapy (NACT), the time between diagnosis of the tumor and HIPEC and the time between NACT and HIPEC (Table 1). In MPC group it was taken into account the time between surgery of the primary tumor and HIPEC (Table 2).
Full table

Full table
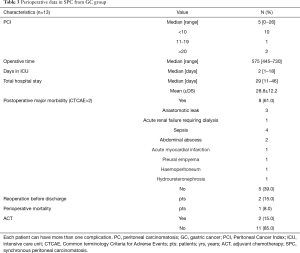
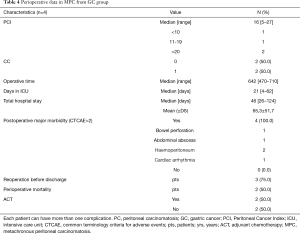
Perioperative data analyzed were: operative time, number of days in intensive care unit (ICU), total hospital stay, postoperative major complications (only complications with CTCAE “Common Terminology Criteria for Adverse Events” >2 were considered), number of patients who underwent reoperation before discharge, perioperative mortality and number of patients who underwent adjuvant chemotherapy (Tables 3,4). Peritoneal Cancer Index (PCI, according to Sugarbacker’s classification) at HIPEC time is reported for SPC and MPC. Every patient underwent pre-operative images (computed tomography, PET, magnetic resonance, EUS) and staging laparoscopy: the peritoneal disease was debulkable and no distant metastases were present in all cases. CRS was performed removing all peritoneum and visceral organs involved. HIPEC was performed according to the coliseum technique for 90 minutes at a temperature of 39–42 °C. One inflow and four outflow catheters were placed with the open abdomen that was partially closed with a surgical adhesive drape performing a “closed-HIPEC with open abdomen technique” and using Belmont® Hyperthermia Pump as perfusion pump. Chemotherapic regimens were cisplatin and paclitaxel or mitomycin-C (MMC) and cisplatin. Afterward, the perfusate was drained and the reconstructive time was performed. The completeness of resection (CC) was graded by the surgeon at the conclusion of the procedure according to the Sugarbacker classification: CC0—complete cytoreduction of all visible disease; CC1—minimal residual disease with nodules less than 2.5 cm; CC2—residual disease with nodules of 2.5 to 2.5 cm; and CC3—residual disease with nodules greater than 2.5 cm. CC is reported for MPC group, while for SPC group all patients were CC0.
Full table
Full table
Postoperative data such as date of recurrence, date of death and date of last follow up were sought.
Statistical analysis
Continuous and categorical variables including frequencies and percentages for categorical data were reported in Tables 1-4. DFS and OS were calculated in the two groups (SPC and MPC) as the interval between the date of CRS and HIPEC and the date of last follow-up or death for OS and the date of last follow-up or the date of recurrence for DFS. The curves of DFS and OS were analyzed using the Kaplan-Meier method, and survival estimates were compared using the log-rank test. Continuous variables were compared with the t-test method. Statistical significance was defined as P value <0.05. All analysis was performed using Graph Pad Prism 7.
Results
A total of 17 patients with GC undergoing CRS+HIPEC were included in the study. 13 patients (77%) have SPC and 4 (23%) have MPC. Groups were comparable in terms of age and gender.
In SPC group all patients had a staging laparoscopy. All patients of SPC group were subject to neoadjuvant chemotherapy and all patients of MPC group underwent adjuvant chemotherapy after surgery of the primary tumor (Tables 1,2). The mean total PCI was 8.5 (SD±8.4). The mean PCI was 3.75 (SD±4.9) in SPC group and 16 (SD±9.5) in MPC group (P=0.003) (Table 3,4). Mean follow up time was 9 months (SD±9.5).
In SPC group 11 patients (85%) received cisplatin (mean dosage 175.9 mg) and paclitaxel (mean dosage 302.8 mg), while 2 patients (15%) had MMC (mean dosage 27.5 mg) and cisplatin (mean dosage 173 mg). In MPC group for 3 patients (75%) cisplatin (mean dosage 150.3 mg) and paclitaxel (mean dosage 263 mg) were used and for 1 patient (25%) MMC (dosage 26 mg) and cisplatin (dosage 163 mg).
In SPC group for all patients CC0 was reached after CRS, while in MPC group for 2 patients (50%) CC1 was obtained after CRS.
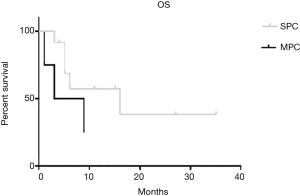
The median OS for SPC group is 16 months and for MPC group is 6 months. There are not any statistically significant differences in OS between the two groups (P=0.189) (Figure 1). DFS for SPC group is 11 months and for MPC is 2 months (P=0.156) (Figure 2).
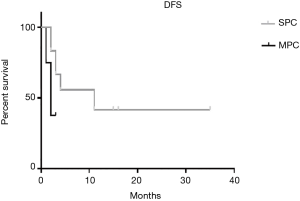
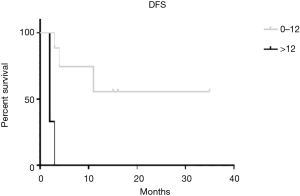
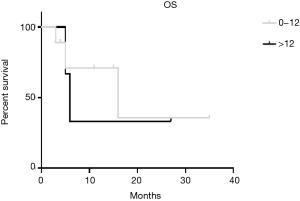
By using the PCI cut-off of 12 (6) we divided patients in the SPC group between PCI ≥12 and PCI <12 founding a statistical significant difference in DFS (P=0.001) (Figure 3). In terms of OS we found a better result in PCI <12 group, but without a statistical significance (16 months in PCI<12 group vs. 6 months in PCI ≥12 group, P=0.825) (Figure 4).
In MPC group CC0 and CC1 patients did not show significant differences in OS and DFS (OS 2 vs. 9 months (P=0.089), DFS 3 vs. 1.5 months (P=0.157) in CC0 and CC1 respectively).
By stratifying patients according to age (≤60 years old vs. >60 years old) we found a statistically significant better DFS for patients with age of >60 years old than those with ≤60 years old (P=0.016) (Figure 5).

We haven’t found any statistical difference in terms of OS and DFS according to ASA score (DFS ASA1-2 vs. ASA 2-3, P=0.677; OS ASA1-2 vs. ASA 2-3, P=0.416).
Postoperative major morbidity (CTCAE >2) was not significantly different between the two groups: 61% (8 pts) for SPC and 100% (4 pts) for MPC (P=0.139). The most frequent major complications were sepsis (4 pts, 23.5%), abdominal abscess (3 pts, 17.6%) and anastomotic leak (3 pts, 17.6%). Reoperation rate was 15% (2 pts) for SPC (both patients were reoperated for sepsis in anastomotic leak) and 75% (3 pts) for MPC (two patients were reoperated for haemoperitoneum and one for abdominal abscess) (P=0.052). Perioperative mortality was 8% (1 pts) for SPC and 50% (2 pts) for MPC (P=0.120). Mean hospital stay was significantly lower in SPC group (28.8 vs. 65.3 days P=0.024).
Discussion
Despite high level of evidence data supporting the use of CRS + HIPEC for treating advanced GC with or without PC, it is still not accepted as standard treatment, likely because GC is still associated with a poor prognosis, even without peritoneal disease (7-10). In analyzing data about CRS + HIPEC in advanced GC it is important to discriminate whether it is used as a prophylactic treatment, or for the management of primary gastric cancer with SPC or for the management of MPC. The most attractive use of HIPEC in GC would be in an adjuvant setting after a curative surgical resection in patients with a high risk of peritoneal recurrence (prophylactic HIPEC). Randomized trials and meta-analysis were performed to study the results of prophylactic HIPEC in patients with locally advanced GC and reported that HIPEC was associated with a significant improvement in the survival rate and in peritoneal recurrence (1,6,8,11-13).
In recent years some randomized trials and meta-analysis have been conducted also to analyze the survival benefits of CRS plus HIPEC compared with the standard of care for patients with synchronous PC from GC. However great heterogeneity exists with respect to the technique, drugs used and their dosage, the duration of HIPEC and the intraperitoneal temperature achieved (14).
The first randomized phase III trial about SPC from GC was reported by Yang et al. (15). The 3-year survival in the CRS + HIPEC arm was 5.9% compared to 0% in the CRS alone arm. Patients treated with CRS + HIPEC had a significantly higher median survival compared to those treated by CRS alone (11 vs. 6.5 months, P=0.04). The authors reported a 70% improvement in the median survival (15). A systematic review of ten published studies comprising 441 patients who underwent CRS + HIPEC for PC from GC reported a 5-year survival of 13%. The median OS was 7.9 months, which increased to 15 months for CC0 (16). A meta-analysis by Coccolini et al. (8) reported that the 3-year mortality and the peritoneal recurrence in patients with established PC was significantly lower in the CRS + HIPEC group (OR =0.25 and 0.29 respectively, P=0.006 for peritoneal recurrence) when compared to the surgery alone group. In a recent retrospective matched pairs-analysis by Boerner et al. (3) the 1-year and 3-years survival of patients who did not receive HIPEC but only gastrectomy were 30% and 0%, in contrast to the 70% and 24% of 1- and 3-year survival in the CRS + HIPEC group (P=0.004). An ongoing phase III randomised European multicentre study (GASTRICHIP) is evaluating the role of HIPEC in patients with GC who have either serosal infiltration and/or lymph nodal involvement and/or positive peritoneal cytology treated by a curative gastrectomy (17).
Results reported in the present study are comparable with the literature with an OS of 16 months and a DFS of 11 months in SPC group.
Currently the treatment of MPC from aggressive cancer, like gastric or pancreatic cancer, has not been reported as successful, unlike in colorectal and appendiceal malignancy (5). Then only few authors take into account CRS+HIPEC for treatment of MPC from GC. Nevertheless although the small sample size and considering the significantly higher PCI in MPC respect to the SPC group (median PCI was 3.75 (SD±4.9) for SPC group and 16 (SD±9.5) for MPC, P=0.003) and the CC1 status of 50% of patients in MPC group, we found an OS of 6 months and a DFS of 2 months in MPC group.
Established that CRS + HIPEC is a safe and effective treatment for advanced GC with or without PC, a proper patient selection had to occur. The first and the most important prognostic indicator is the completeness of cytoreduction score. In a recent meta-analysis by Coccolini et al. (18) 1-, 2-, 3- and 5-year survival is incremented by CC0-CC1 cytoreduction and CC0 cytoreduction increases 1 and 3-year survival rate if compared with CC1 (RR =2.28–6.36). In our study the CC0 status has been reached in all patients of SPC group, but only in 50% of patients in MPC group. No differences in DFS and in OS between CC0 and CC1 in MPC group were observed, probably due to the small sample size.
The second crucial prognostic indicator is the PCI that is used to determine if the surgical intervention should continue to be an attempt to cure or for palliation only. As for the CC, the higher the grade of disease and the higher the aggressive nature of the malignant process, the lower the PCI must be in order to achieve long-term success (5). The effective PCI cut-off to obtain the best survival benefit in GC is 12 (19) as we also have found in our study. Patients with PCI <12 in SPC group shown better outcomes in DFS (P=0.001).
Regarding morbidity and mortality of the procedure of CRS + HIPEC, a meta-analysis by Tristan reported a similar mortality in the two groups (CRS + HIPEC vs. surgery alone) and no statistical differences of bowel fistula, pancreatic fistula and anastomotic leak. Meta-analysis showed a significant increase of intra-abdominal abscess and neutropenia rates in the HIPEC group (19). We have not found any statistically significant differences between major morbidity in the two groups analyzed, even if there was an increased rate of major complication in MPC group vs. SPC group (61% SPC, 100% MPC, P=0.139). The same tendency can be observed in terms of reoperation rates (15% SPC, 75% MPC, P=0.052) and for perioperative mortality (8% SPC, 50% MPC, P=0.120). However a significant difference in mean of days of hospital stay was found (28.8 vs. 65.3 days in SPC and MPC respectively, P=0.001). Probably these differences are due to the different complexity of the CRS in the two groups, according to the higher mean PCI in MPC group.
An interesting result was found in older patients: patients aged >60 years showed a higher DFS compared to the younger patients (≤60 years) (P=0.016). This result can probably be due to the higher aggressiveness of the disease in the younger group. However comorbidities and ASA score does not seems to affect OS and DFS (DFS ASA1-2 vs. ASA 2−3, P=0.677; OS ASA 1−2 vs. ASA 2−3, P=0.416).
Differently from the other studies in literature we tested a new chemotherapy regimen for HIPEC: Cisplatin and Paclitaxel were used for most patients because it seems to have a favorable pharmacokinetic profile and high drug concentrations can be achieved in peritoneal tissue with low systemic exposure (20). With this drugs regimen we have reached promising result in OS and DFS in SPC group.
Despite the promising outcomes especially for SPC group, the small sample size and the lack of uniformity in patients of the two groups in terms of NACT regimen and of mean PCI can be considered as limitations of this study. Furthermore in the two groups only two patients were subject to adjuvant chemotherapy after CRS + HIPEC, independently from their general conditions, and this fact can negatively influence the OS and the DFS. This situation can be resolved adopting a standardized multidisciplinary approach shared between surgeons and oncologists.
Conclusions
CRS + HIPEC with cisplatin and paclitaxel is a safe and effective treatment for GC with PC compared to traditional treatments, especially in patients with PCI <12 and in older patients, although with a high incidence of complications, especially in MPC group.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical statement: The study was approved by provincial ethics board of Bergamo (NO. Ch1BG.01) and informed consent was taken from all the patients.
References
- Seshadri RA, Glehen O. The Role of Hyperthermic Intraperitoneal Chemotherapy in Gastric Cancer. Indian J Surg Oncol 2016;7:198-207. [Crossref] [PubMed]
- Van Cutsem E, Sagaert X, Topal B, et al. Gastric cancer. Lancet 2016;388:2654-64. [Crossref] [PubMed]
- Boerner T, Graichen A, Jeiter T, et al. CRS-HIPEC prolongs survival but is not curative for patients with peritoneal carcinomatosis of gastric cancer. Ann Surg Oncol 2016;23:3972. [Crossref] [PubMed]
- Coccolini F, Montori G, Ceresoli M, et al. Advanced gastric cancer: what we know and what we still have to learn. World J Gastroenterol 2016;22:1139-59. [Crossref] [PubMed]
- Sugarbaker PH. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of gastrointestinal cancers with peritoneal metastases: progress toward a new standard of care. Cancer Treatment Reviews 2016;48:42-9. [Crossref] [PubMed]
- Coccolini F, Celotti A, Ceresoli M, et al. Hyperthermic intraperitoneal chemotherapy (HIPEC) and neoadjuvant chemotherapy as prophylaxis of peritoneal carcinosis from advanced gastric cancer – effects on overall and disease free survival. J Gastrointest Oncol 2016;7:523-9. [Crossref] [PubMed]
- Chia CS, You B, Decullier E, et al. Patients with peritoneal carcinomatosis from gastric cancer treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: is cure a possibility? Ann Surg Oncol 2016.23:1971-9.
- Coccolini F, Cotte E, Glehen O, et al. Intraperitoneal chemotherapy in advanced gastric cancer. Meta-analysis of randomized trials. Eur J Surg Oncol 2014;40:12-26. [Crossref] [PubMed]
- Spiliotis J, Halkia E, De Bree E. Treatment of peritoneal surface malignancies with hyperthermic intraperitoneal chemotherapy - current perspective. Curr Oncol 2016;23:e266-e275. [Crossref] [PubMed]
- Roviello F, Caruso S, Neri A, et al. Treatment and prevention of peritoneal carcinomatosis from gastric cancer by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: overview and rationale. Eur J Surg Oncol 2013;39:1309-16. [Crossref] [PubMed]
- Coccolini F, Catena F, Glehen O, et al. Effect of intraperitoneal chemotherapy and peritoneal lavage in positive peritoneal cytology in gastric cancer. Systematic review and meta-analysis. Eur J Surg Oncol 2016;42:1261-7. [Crossref] [PubMed]
- Sun J, Song Y, Wang Z. benefits of hyperthermic intraperitoneal chemotherapy for patients with serosal invasion in gastric cancer: a meta-analysis of randomized controlled trials. BMC Cancer 2012;12:526. [Crossref] [PubMed]
- Huang JY, Xu Y, Sun Z, et al. Comparison different methods of intraoperative and intraperitoneal chemotherapy for patients with gastric cancer: a meta-analysis. Asian Pac J Cancer Prev 2012;13:4379-85. [Crossref] [PubMed]
- Coccolini F, Ansaloni L, Corbella D, et al. Criticalities in randomized controlled trials on HIPEC for ovarian cancer. World Journal of Obstetrics and Gynecology 2013;2:124-8. [Crossref]
- Yang XJ, Huang CQ, Suo T, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Ann Surg Oncol 2011;18:1575-81. [Crossref] [PubMed]
- Gill RS, Al-adra D, Nagendran J, et al. Treatment of gastric cancer with peritoneal carcinomatosis by cytoreductive surgery and HIPEC: A systematic review of survival, mortality, and morbidity. J Surg Oncol 2011;104:692-8. [Crossref] [PubMed]
- Available online: https://clinicaltrials.gov/ct2/show/NCT01882933
- Coccolini F, Catena F, Glehen O, et al. Complete versus incomplete cytoreduction in peritoneal carcinosis from gastric cancer, with consideration to PCI cut-off. Systematic review and meta-analysis. Eur J Surg Oncol 2015;41:911-9. [Crossref] [PubMed]
- Yan TD, Black D, Sugarbaker PH, et al. A systematic review and meta-analysis of the randomized controlled trials on adjuvant intraperitoneal chemotherapy for resectable gastric cancer. Ann Surg Oncol 2007;14:2702-13. [Crossref] [PubMed]
- Ansaloni L, Coccolini F, Morosi L, et al. Pharmacokinetics of concomitant cisplatin and paclitaxel administered by hyperthermic intraperitoneal chemotherapy to patients with peritoneal carcinomatosis from epithelial ovarian cancer. Br J Cancer 2015;112:306-12. [Crossref] [PubMed]


