Chemotherapy in patients with hepatobiliary cancers and abnormal hepatic function
Introduction
Hepatocellular carcinoma (HCC) and biliary tract cancer are among the leading causes of cancer death in the United States with an estimated 39,230 new cases and 27,170 deaths in both men and women in 2016 (1). The combination of cisplatin + gemcitabine represents the standard first-line therapy in advanced biliary cancer (ABC) based on survival advantages conferred over gemcitabine alone in the phase III ABC-02 trial (2). In the event cisplatin is contraindicated, i.e., renal dysfunction, alternative first-line regimens exist. Gemcitabine + capecitabine has demonstrated activity in phase II trials in ABC (3,4). Several phase II trials have similarly shown responses to gemcitabine + 5-fluorouracil (5-FU) + leucovorin (LV)] in unresectable or metastatic biliary cancer (5-7). Another option includes gemcitabine + oxaliplatin, which has been investigated in phase II trials, though this combination is often associated with increased myelosuppression and neurotoxicity compared to cisplatin + gemcitabine (8-10). For those who have progressed through first-line chemotherapy, best supportive care (BSC) remains an acceptable standard in ABC though second-line chemotherapy may offer some benefits (11). Capecitabine monotherapy, 5-FU/LV + oxaliplatin (FOLFOX), 5-FU/LV + irinotecan (FOLFIRI) represent options with activity in the second-line treatment of ABC (12-15).
In unresectable or metastatic HCC, sorafenib has been established as a first-line standard therapy given its superiority over BSC in 2 separate phase III trials in patients with Child-Pugh A liver function (16,17). While previously considered a standard therapy in inoperable HCC, doxorubicin demonstrated significant toxicities (neutropenia and cardiotoxicity) that outweighed its modest benefits in this population (18). Moreover, FOLFOX represents a more preferred first-line option over doxorubicin given the improved progression-free survival (PFS) and overall response rates (ORRs) seen with FOLFOX4 vs. single-agent doxorubicin in a phase III trial involving Asian patients with advanced HCC (19).
The majority of patients with HCC and biliary cancer present with advanced disease (20,21). The intrinsic liver dysfunction associated with hepatobiliary cancer often presents a challenge to the administration of the aforementioned systemic therapies in the treatment of advanced disease. In addition, chemotherapy and targeted therapy-induced hepatotoxicity can further complicate the treatment in these patients. The potential toxic effects of chemotherapy (including the majority of agents used in the treatment of advanced hepatobiliary cancer) on the liver have been extensively reviewed (22,23). Instead, this review will focus on dosage considerations for chemotherapy and targeted therapy in patients with abnormal hepatic function. Specifically, we review available clinical data that offer recommendations for dosing strategies, in the setting of liver dysfunction, of systemic therapies used in treating advanced hepatobiliary cancer.
Chemotherapy and targeted therapy in patients with liver dysfunction
There is a growing body of clinical evidence to recommend dosing guidelines based on serum liver biochemical tests involving agents used in the treatment of advanced hepatobiliary cancer in patients with liver dysfunction (Tables 1-3).
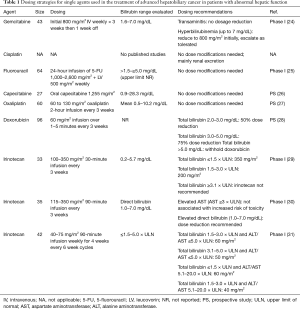
Full table
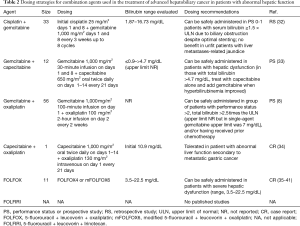
Full table

Full table
Gemcitabine
An early phase I trial investigated gemcitabine at a starting dose of 800 mg/m2 30-minute infusion every week for 3 weeks followed by 1 week off in 43 patients stratified by aspartate aminotransferase (AST) ≥2 times the upper limit of normal (ULN), total bilirubin of 1.6–7.0 mg/dL with any AST level, and serum creatinine of 1.6–5.0 mg/dL (24). The most frequent dose-limiting toxicity (DLT) was hyperbilirubinemia (≥1.5 times baseline) seen in 7 patients in the cohort with a median bilirubin of 2.7 mg/dL (range, 1.7–5.7 mg/dL). Hyperbilirubinemia was transient and often lasting <1 week in the majority of affected patients. In this same cohort, 3/8 patients experienced DLTs at the 800 mg/m2 dosing level while 8/10 experienced DLTs at the 950 mg/m2 dosing level. There were no apparent differences in pharmacokinetics (PKs) compared to historical controls. The investigators concluded that no dose reductions of gemcitabine are required in those with elevated transaminases or creatinine, but in those with elevated bilirubin, gemcitabine should be reduced to 800 mg/m2 initially with subsequent escalation in the absence of DLTs (Table 1). In a recent retrospective case series, all 7 patients with total bilirubin ≥4.5 mg/dL received full doses of 1,000 mg/m2 gemcitabine safely without further worsening of liver function and only 1 of 7 developed significant thrombocytopenia necessitating a dose to be held (42). The authors recommended that no initial dose reduction is needed in patients with impaired liver function receiving gemcitabine though close monitoring is preferred.
Cisplatin
Cisplatin and carboplatin are primarily excreted by the kidneys and do not require dose modifications in patients with abnormal hepatic function. No formal studies exist but a pilot study investigating cisplatin 75 mg/m2 every 21 days + vinorelbine 20 mg/m2 on days 1 and 8 every 21 days in 11 patients with severe liver dysfunction caused by metastatic breast cancer with serum bilirubin >1.5 times the ULN showed improvement in liver function tests in 10 patients by day 40 of treatment (43). No dose modifications were needed and there were no significant adverse events (AEs) related to hepatotoxicity.
Fluorouracil
A phase I trial stratified 64 patients to receive weekly 24-hour infusions of 1,000–2,600 mg/m2 5-FU + LV 500 mg/m2 into 3 cohorts: elevated creatinine (>1.5 mg/dL) but normal bilirubin, bilirubin >1.5 mg/dL but <5.0 mg/dL with normal creatinine, and bilirubin ≥5.0 mg/dL with normal creatinine (25). Across all 3 cohorts, there was no apparent difference in likelihood of experiencing DLTs and infusional 5-FU (2,600 mg/m2) can be safely administered without dosage adjustments (Table 1). There was no significant relationship between serum bilirubin or creatinine and 5-FU clearance.
Capecitabine
An open-label, multicenter prospective study investigated PK parameters following administration of a single oral dose of capecitabine (1,255 mg/m2) in 14 patients with normal liver function and 13 patients with liver dysfunction secondary to liver metastases (mean bilirubin 6.5 mg/dL; range, 0.9–28.3 mg/dL) and demonstrated that liver dysfunction had no clinically significant influence on the PK parameters of capecitabine and its metabolites (26). No clinically significant differences in AEs were observed between patients with or without hepatic impairment (Table 1). Hyperbilirubinemia is a known capecitabine-associated adverse event, even in patients with normal hepatic function. We have therefore replaced this agent, when feasible, with 5-FU infusional therapy in the settings of patients with hepatic dysfunction.
Oxaliplatin
A dose-escalation trial enrolled 60 patients with advanced solid tumors and normal-severe hepatic dysfunction (severe defined as bilirubin >3.0 mg/dL) to receive 60 to 130 mg/m2 oxaliplatin 2-hour infusion every 3 weeks (27). No DLTs were observed at the maximum tolerated dose (MTD) defined as 130 mg/m2 oxaliplatin in the cohort with severe liver dysfunction. Additionally, abnormal liver function did not correlate with platinum clearance. The investigators concluded that full doses of single-agent oxaliplatin can be safely administered in patients with liver dysfunction without the need for dosage adjustments (Table 1).
Doxorubicin
Although largely replaced by other standard therapies in the treatment of advanced HCC, doxorubicin has been associated with increased risk of toxicities in those with impaired liver function (Table 1). An early study enrolled 96 patients with predominantly pretreated malignancies to receive a planned dose of doxorubicin 60 mg/m2 infusion over 1–5 minutes every 3 weeks (28). Notably, 8 patients with significant liver dysfunction experienced increased toxicities of severe mucositis (3 patients or 38%) and severe pancytopenia (all 8 patients). Liver dysfunction was shown to delay clearance of doxorubicin and increase plasma levels of doxorubicin and its metabolites by 4–5-fold. The investigators recommended a 50% dose reduction for total bilirubin of 2.0–3.0 mg/dL, a 75% dose reduction for bilirubin of 3.0–5.0 mg/dL, and to withhold doxorubicin for bilirubin >5.0 mg/dL. However, others have contended that the small number of patients with liver dysfunction in this study does not justify an indiscriminate dose reduction and argue for dose modification of doxorubicin only in the setting of total bilirubin >3.0 mg/dL (44). Given the lack of known activity of doxorubicin in cholangiocarcinoma and its limited activity in HCC, the authors recommend against its use in patients with severe hepatic dysfunction. Patients with HCC and bilirubin levels exceeding 3 mg/dL have not been shown to derive a clinical benefit from cytotoxic chemotherapy, specifically doxorubicin. Such patients may be best addressed with palliative intent.
Irinotecan
Several phase I trials have investigated the safety of irinotecan and its effects on PKs in patients with abnormal hepatic function (Table 1). One phase I trial enrolled 33 patients with refractory solid tumors and assigned irinotecan 350 mg/m2 30-minute infusion every 3 weeks to those with normal bilirubin and total bilirubin 1.1–1.5 times the ULN (29). Patients with bilirubin 1.51–3.0 times the ULN and ≥3.1 times the ULN were assigned to starting doses of 175 mg/m2 and 100 mg/m2 irinotecan, respectively. The recommended dose of irinotecan was 350 mg/m2 in those with bilirubin ≤1.5 times the ULN. The recommended dose of irinotecan in those with bilirubin 1.5–3.0 times the ULN was 200 mg/m2. Due to rapid progression of hepatic metastases and poor PS in patients with total bilirubin ≥3.1 times the ULN, dose escalation could not be carried out and dosing of irinotecan could not be recommended. Hyperbilirubinemia and elevated alkaline phosphatase (ALP) were associated with an exponential decrease in clearance of irinotecan and its metabolites (likely due to decreased biliary excretion).
A separate phase I trial enrolled 35 patients with varying degrees of liver dysfunction to receive 115–350 mg/m2 of irinotecan 90-minute infusion every 3 weeks (30). In the cohort of patients with AST ≥3 times the ULN with normal bilirubin and serum creatinine, there were no DLTs at the 225 mg/m2 dosing level. However, patients with direct bilirubin 1.0–7.0 mg/dL and any level of AST with normal serum creatinine (<1.6 mg/dL) experienced DLTs at 145 mg/m2 of irinotecan and experienced higher relative exposure to irinotecan and SN-38 due to reduced clearance. This study confirmed that elevated bilirubin is associated with increased risk of toxicity from irinotecan and a dose reduction is recommended.
Another phase I trial investigated 40–75 mg/m2 of irinotecan 90-minute infusion weekly for 4 weeks every 6 week cycles in 42 patients with varying degrees of hepatic dysfunction (31). The recommended dose of weekly irinotecan was 60 mg/m2 for those with total bilirubin 1.5–3.0 times the ULN and alanine aminotransferase (ALT) to AST ratio (ALT/AST) ≤5.0 times the ULN, 50 mg/m2 with total bilirubin 3.1–5.0 times the ULN and ALT/AST ≤5.0 times the ULN, 60 mg/m2 with total bilirubin ≤1.5 times the ULN and ALT/AST 5.1–20.0 times the ULN, and 40 mg/m2 with total bilirubin 1.5–3.0 times the ULN and ALT/AST 5.1–20.0 times the ULN. Increased serum bilirubin was a relatively strong indicator of reduced clearance of irinotecan and SN-38, more so than other biochemical markers of liver function given the eventual plateau of irinotecan and SN-38 area under the curve (AUC) values with increasing AST.
Given the limited safety data with every 3 week irinotecan dosing with bilirubin levels >3 mg/dL, the authors do not recommend this schedule in this patient population. If irinotecan is to be considered in patients with bilirubin levels between and 3 and 5 mg/dL, we recommend the use of a weekly regimen at 50 mg/m2/week as described by Schaaf et al. (31). Of note, no dedicated studies have been reported with FOLFIRI in patients with severe hepatic dysfunction to make firm recommendations.
Cisplatin + gemcitabine
A retrospective study of planned cisplatin (25 mg/m2 days 1 and 8) gemcitabine (1,000 mg/m2 days 1 and 8) every 3 weeks up to 8 cycles in the first-line treatment of ABC in 33 patients [performance status (PS) 0-1] with serum bilirubin ≥1.5 times the ULN due to biliary obstruction despite optimal stenting identified comparable rates of abnormal liver function tests (drug-related) to the non-jaundiced cohort in the ABC-02 trial (32). Full-dose cisplatin + gemcitabine was safely administered in the majority of patients with biliary obstruction-related jaundice (70%) and resulted in normalization of bilirubin in 68% of patients in this cohort (Table 2). On the contrary, cisplatin + gemcitabine did not appear to provide benefits in patients with poor PS and liver metastases-related jaundice when compared to those with biliary obstruction-related jaundice.
Gemcitabine + capecitabine
A recent prospective study investigated gemcitabine 1,000 mg/m2 30-minute infusion on days 1 and 8 + capecitabine 650 mg/m2 oral twice daily on days 1–14 every 21 days in 12 patients with unresectable or metastatic pancreatic or biliary cancer (33). Eight patients had varying degrees of hepatic dysfunction (2 with total bilirubin of 0.9–2.3 mg/dL, 3 with total bilirubin of 2.3–4.7 mg/dL, and 3 with total bilirubin >4.7 mg/dL) due to liver metastases (50%) or extrahepatic cholestasis from tumor compression (50%). There was no significant correlation between hepatic function cohort and DLT leading to dose reduction or withholding of a dose (P=0.55). However, hepatic dysfunction was associated with reduced clearance of both gemcitabine and capecitabine and limited activity of gemcitabine due to decreased intracellular activation. In short, standard dosing of gemcitabine + capecitabine could be safely administered in patients with hepatic dysfunction though it was recommended that patient’s with total bilirubin >4.7 mg/dL should be initially treated with single-agent capecitabine with gemcitabine added once hyperbilirubinemia improves (Table 2).
Gemcitabine + fluorouracil
The original phase I studies investigating the combination of gemcitabine + 5-FU in refractory or unresectable solid tumors enrolled patients with adequate hepatic function (as restrictive as total bilirubin ≤1.5 mg/dL) and renal function (45-47). To our knowledge, there are no formal studies to date evaluating the safety of gemcitabine + 5-FU in patients with liver dysfunction; recommendations for dosing strategies in this population are similarly lacking.
Gemcitabine + oxaliplatin
A prospective study enrolled 56 patients with unresectable or metastatic ABC to receive gemcitabine 1,000 mg/m2 100-minute infusion on day 1 followed by oxaliplatin 100 mg/m2 2-hour infusion on day 2 every 2 weeks (8). A group of 23 patients were defined by PS >2, total bilirubin >2.5 times the ULN, and/or having received prior chemotherapy (group B); 13 patients in this group (57%) had total bilirubin ≥2.5 times the ULN. Tolerability to gemcitabine + oxaliplatin in group B was not significantly different from group A patients (normal counterparts), and the investigators concluded that this combination is safe in this cohort with abnormal liver function (Table 2). Formal dosing recommendations have not been evaluated in a dedicated study involving gemcitabine + oxaliplatin in the setting of liver dysfunction, however.
Capecitabine + oxaliplatin
Similarly, the combination of capecitabine + oxaliplatin has not been formally studied in patients with hepatic dysfunction in the phase I setting. A case report describing a patient with abnormal liver function secondary to metastatic gastric cancer (initial serum bilirubin 10.9 mg/dL) reported that capecitabine 1,000 mg/m2 oral twice daily on days 1–14 + oxaliplatin 130 mg/m2 intravenous (IV) on day 1 every 21 days can be safely administered in the setting of hepatic impairment (34). After 2 cycles of therapy, total bilirubin and alkaline phosphatase demonstrated near-normalization and no treatment-related grade ≥3 toxicities occurred during first 4 cycles of therapy.
FOLFOX
Several case reports involving 11 separate patients with metastatic colorectal cancer have shown that the administration of FOLFOX4 or mFOLFOX6 is safe and tolerated in patients with severe hepatic dysfunction (35-41). Total bilirubin levels ranged from 3.5–22.5 mg/dL and treatment with FOLFOX often resulted in dramatic improvements in hyperbilirubinemia as early as 1–2 cycles of therapy without significant toxicities (Table 2). Many of these patients also achieved durable responses in their metastatic disease following treatment with FOLFOX.
FOLFIRI
In a single-institution retrospective study, 156 patients with metastatic colorectal cancer treated with first-line FOLFIRI-based regimens were evaluated for liver toxicity (48). The majority of patients had liver involvement (64.74%) during the initiation of first-line therapy. Levels of AST, ALT, and alkaline phosphatase (AE) were all found to be significantly increased during first 3 months of treatment and the calculated R ratio was 3.96 (3.25–4.51). Twenty-five patients (16.02%) experienced course delays, 12 (7.69%) needed a dose reduction, and 3 (1.92%) stopped therapy due to significant liver toxicity. Of note, this study described patterns of liver toxicity in patients treated with a FOLFIRI-based backbone of chemotherapy. Dedicated safety evaluations of an exclusive FOLFIRI regimen in the phase I setting and formal dosing recommendations in patients with liver dysfunction are lacking.
Sorafenib
A phase I study (CALGB 60301) enrolled 138 patients with various solid and hematologic malignancies and stratified patients to receive doses of oral sorafenib that ranged from 200 mg every other day to 400 mg twice daily depending on increasing severity of hepatic dysfunction or renal dysfunction (49). A single oral dose of 400 mg sorafenib was administered on day 1 to evaluate PKs, and one cohort was defined by normal renal and liver function. There was no significant association between AUCs of sorafenib or its major metabolite and cohort (P>0.05). In the hepatic dysfunction cohort, DLTs included total bilirubin ≥1.5 times the baseline (n=10), grade 3 diarrhea (n=1), grade 3 fatigue (n=1), grade 3 fatigue and reduction in creatinine clearance >10 mL/min (n=1), and grade 3 hypertension (n=1). Accordingly, the investigators recommended a dose of sorafenib 400 mg twice daily in those with total bilirubin > ULN but ≤1.5 times the ULN and/or AST > ULN, 200 mg twice daily for total bilirubin >1.5 times the ULN to ≤3 times ULN with any AST, not recommended for total bilirubin >3 times the ULN to 10 times the ULN with any AST, and 200 mg daily for albumin <2.5 mg/dL with any bilirubin/AST (Table 3).
The prospective Global Investigation of Therapeutic Decisions in Hepatocellular Carcinoma and of Its Treatment with Sorafenib (GIDEON) nonrandomized observational study enrolled >3,000 patients with unresectable HCC treated with sorafenib ± transarterial chemoembolization (TACE) and identified an overall survival of 21.6 months (95% confidence interval 18.0-not reached) with concomitant sorafenib and TACE vs. 9.7 months (95% confidence interval 9.2–10.4) in those not treated concomitantly (50). In a United States regional analysis, 543 patients were stratified into those who received TACE prior to initiating sorafenib (group A, n=158), underwent TACE after initiating sorafenib (group B, n=29), received TACE concurrently with sorafenib (group C, n=38), and received sorafenib without TACE (n=318). Notably, at least 35% of patients in all groups had Child-Pugh B or C liver function at the start of sorafenib therapy (51). The majority of patients in all groups received full-dose sorafenib initially, and the investigators concluded that these observations reflect the high degree of comfort in using sorafenib and TACE in those with abnormal liver function in real-life clinical settings.
Conclusions
Treatment standards currently favor combination chemotherapy and targeted therapy, i.e. sorafenib, in ABC and advanced HCC, respectively. In second-line settings or those with contraindications to first-line agents, conventional cytotoxic agents (monotherapy or combination therapy) have demonstrated activity in advanced HCC and biliary tract cancer. In populations with hepatobiliary cancer and abnormal liver function, an appropriate strategy providing effective yet safe administration of such systemic agents needs to be strongly considered.
The majority of clinical trials (phase I–III) have historically excluded patients with predefined hepatic impairment, i.e., abnormal serum liver function tests. Very little is often known regarding the appropriate starting doses of chemotherapy in patients with liver dysfunction. As a result, dose reductions commonly performed in clinical practice are often empiric with re-escalation of dose in the absence of DLTs. There is a growing body of phase I studies providing data on PKs and dosing strategies of chemotherapy in those with hepatic dysfunction. However, formal phase I studies on PKs and dosing recommendations of several combination regimens in those with hepatobiliary cancer and impaired liver function are still lacking. Furthermore, available dosing strategies often focus on initial dosing recommendations in those with abnormal liver function tests. Guidelines on how long to withhold a dose and what dose to restart therapy are vague and such decisions are often empirically performed by the clinician.
Further dedicated phase I studies offering specific dosing strategies for agents routinely used for treating advanced hepatobiliary cancer in those with hepatic dysfunction are warranted, particularly in combination regimens when PKs and tolerability may be influenced by drug-drug interactions and liver metabolism. Retrospective analyses and case series may also provide additional information on safety and dosing strategies in those with abnormal liver function. Future studies should also incorporate other measures of liver function including AST, ALT, AP, and albumin beyond the standard total bilirubin to assess safety and dosing considerations in those with liver disease receiving chemotherapy. For now, an appropriate strategy for effective yet safe dosing of systemic therapies in patients with liver dysfunction will need to balance available safety data from clinical studies on one hand and a risks and benefits discussion between the clinician and patient on the other.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273-81. [Crossref] [PubMed]
- Knox JJ, Hedley D, Oza A, et al. Combining gemcitabine and capecitabine in patients with advanced biliary cancer: a phase II trial. J Clin Oncol 2005;23:2332-8. [Crossref] [PubMed]
- Riechelmann RP, Townsley CA, Chin SN, et al. Expanded phase II trial of gemcitabine and capecitabine for advanced biliary cancer. Cancer 2007;110:1307-12. [Crossref] [PubMed]
- Alberts SR, Al-Khatib H, Mahoney MR, et al. Gemcitabine, 5-fluorouracil, and leucovorin in advanced biliary tract and gallbladder carcinoma: a North Central Cancer Treatment Group phase II trial. Cancer 2005;103:111-8. [Crossref] [PubMed]
- Gebbia V, Giuliani F, Maiello E, et al. Treatment of inoperable and/or metastatic biliary tree carcinomas with single-agent gemcitabine or in combination with levofolinic acid and infusional fluorouracil: results of a multicenter phase II study. J Clin Oncol 2001;19:4089-91. [Crossref] [PubMed]
- Murad AM, Guimaraes RC, Aragao BC, et al. Phase II trial of the use of gemcitabine and 5-fluorouracil in the treatment of advanced pancreatic and biliary tract cancer. Am J Clin Oncol 2003;26:151-4. [Crossref] [PubMed]
- André T, Tournigand C, Rosmorduc O, et al. Gemcitabine combined with oxaliplatin (GEMOX) in advanced biliary tract adenocarcinoma: a GERCOR study. Ann Oncol 2004;15:1339-43. [Crossref] [PubMed]
- Verderame F, Russo A, Di Leo R, et al. Gemcitabine and oxaliplatin combination chemotherapy in advanced biliary tract cancers. Ann Oncol 2006;17 Suppl 7:vii68-72. [Crossref] [PubMed]
- André T, Reyes-Vidal JM, Fartoux L, et al. Gemcitabine and oxaliplatin in advanced biliary tract carcinoma: a phase II study. Br J Cancer 2008;99:862-7. [Crossref] [PubMed]
- Lamarca A, Hubner RA, David Ryder W, et al. Second-line chemotherapy in advanced biliary cancer: a systematic review. Ann Oncol 2014;25:2328-38. [Crossref]
- He S, Shen J, Sun X, et al. A phase II FOLFOX-4 regimen as second-line treatment in advanced biliary tract cancer refractory to gemcitabine/cisplatin. J Chemother 2014;26:243-7. [Crossref] [PubMed]
- Moretto R, Raimondo L, De Stefano A, et al. FOLFIRI in patients with locally advanced or metastatic pancreatic or biliary tract carcinoma: a monoinstitutional experience. Anticancer Drugs 2013;24:980-5. [Crossref] [PubMed]
- Patt YZ, Hassan MM, Aguayo A, et al. Oral capecitabine for the treatment of hepatocellular carcinoma, cholangiocarcinoma, and gallbladder carcinoma. Cancer 2004;101:578-86. [Crossref] [PubMed]
- Nehls O, Klump B, Arkenau HT, et al. Oxaliplatin, fluorouracil and leucovorin for advanced biliary system adenocarcinomas: a prospective phase II trial. Br J Cancer 2002;87:702-4. [Crossref] [PubMed]
- Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009;10:25-34. [Crossref] [PubMed]
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. [Crossref] [PubMed]
- Lai CL, Wu PC, Chan GC, et al. Doxorubicin versus no antitumor therapy in inoperable hepatocellular carcinoma. A prospective randomized trial. Cancer 1988;62:479-83. [Crossref] [PubMed]
- Qin S, Bai Y, Lim HY, et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol 2013;31:3501-8. [Crossref] [PubMed]
- de Groen PC, Gores GJ, LaRusso NF, et al. Biliary tract cancers. N Engl J Med 1999;341:1368-78. [Crossref] [PubMed]
- Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet 2003;362:1907-17. [Crossref] [PubMed]
- Field KM, Dow C, Michael M, Part I. Liver function in oncology: biochemistry and beyond. Lancet Oncol 2008;9:1092-101. [Crossref] [PubMed]
- Ramadori G, Cameron S. Effects of systemic chemotherapy on the liver. Ann Hepatol 2010;9:133-43. [PubMed]
- Venook AP, Egorin MJ, Rosner GL, et al. Phase I and pharmacokinetic trial of gemcitabine in patients with hepatic or renal dysfunction: Cancer and Leukemia Group B 9565. J Clin Oncol 2000;18:2780-7. [Crossref] [PubMed]
- Fleming GF, Schilsky RL, Schumm LP, et al. Phase I and pharmacokinetic study of 24-hour infusion 5-fluorouracil and leucovorin in patients with organ dysfunction. Ann Oncol 2003;14:1142-7. [Crossref] [PubMed]
- Twelves C, Glynne-Jones R, Cassidy J, et al. Effect of hepatic dysfunction due to liver metastases on the pharmacokinetics of capecitabine and its metabolites. Clin Cancer Res 1999;5:1696-702. [PubMed]
- Synold TW, Takimoto CH, Doroshow JH, et al. Dose-escalating and pharmacologic study of oxaliplatin in adult cancer patients with impaired hepatic function: a National Cancer Institute Organ Dysfunction Working Group study. Clin Cancer Res 2007;13:3660-6. [Crossref] [PubMed]
- Benjamin RS, Wiernik PH, Bachur NR. Adriamycin chemotherapy--efficacy, safety, and pharmacologic basis of an intermittent single high-dosage schedule. Cancer 1974;33:19-27. [Crossref] [PubMed]
- Raymond E, Boige V, Faivre S, et al. Dosage adjustment and pharmacokinetic profile of irinotecan in cancer patients with hepatic dysfunction. J Clin Oncol 2002;20:4303-12. [Crossref] [PubMed]
- Venook AP, Enders Klein C, Fleming G, et al. A phase I and pharmacokinetic study of irinotecan in patients with hepatic or renal dysfunction or with prior pelvic radiation: CALGB 9863. Ann Oncol 2003;14:1783-90. [Crossref] [PubMed]
- Schaaf LJ, Hammond LA, Tipping SJ, et al. Phase 1 and pharmacokinetic study of intravenous irinotecan in refractory solid tumor patients with hepatic dysfunction. Clin Cancer Res 2006;12:3782-91. [Crossref] [PubMed]
- Lamarca A, Benafif S, Ross P, et al. Cisplatin and gemcitabine in patients with advanced biliary tract cancer (ABC) and persistent jaundice despite optimal stenting: Effective intervention in patients with luminal disease. Eur J Cancer 2015;51:1694-703. [Crossref] [PubMed]
- Joerger M, Huitema AD, Koeberle D, et al. Safety and pharmacology of gemcitabine and capecitabine in patients with advanced pancreatico-biliary cancer and hepatic dysfunction. Cancer Chemother Pharmacol 2014;73:113-24. [Crossref] [PubMed]
- Hwang SJ, Park JW, Lee SD, et al. Capecitabine and oxaliplatin (XELOX) for the treatment of patients with metastatic gastric cancer and severe liver dysfunction. Korean J Intern Med 2006;21:252-5. [Crossref] [PubMed]
- Fakih MG. 5-fluorouracil leucovorin and oxaliplatin (FOLFOX) in the treatment of metastatic colon cancer with severe liver dysfunction. Oncology 2004;67:222-4. [Crossref] [PubMed]
- Shimura T, Kataoka H, Hirata Y, et al. Metastatic colorectal cancer with severe liver dysfunction successfully treated using FOLFOX therapy. J Gastrointest Cancer 2011;42:68-72. [Crossref] [PubMed]
- Grenader T, Goldberg A, Gabizon A. Combination therapy with oxaliplatin and 5-fluorouracil in a patient with severe hepatic dysfunction associated with metastatic adenocarcinoma of the large bowel. Anticancer Drugs 2009;20:845-7. [Crossref] [PubMed]
- Elsoueidi R, Craig J, Mourad H, et al. Safety and efficacy of FOLFOX followed by cetuximab for metastatic colorectal cancer with severe liver dysfunction. J Natl Compr Canc Netw 2014;12:155-60. [PubMed]
- Kasi PM, Thanarajasingam G, Finnes HD, et al. Chemotherapy in the Setting of Severe Liver Dysfunction in Patients with Metastatic Colorectal Cancer. Case Rep Oncol Med 2015;2015:420159.
- Roderburg C. Safe use of FOLFOX in two patients with metastatic colorectal carcinoma and severe hepatic dysfunction. Clin Colorectal Cancer 2011;10:E6-9. [Crossref] [PubMed]
- Tural D, Akar E, Ozturk MA, et al. Severe liver dysfunction and safe use of 5-fluorouracil leucovorin and oxaliplatin in one patient with metastatic colorectal carcinoma. J Cancer Res Ther 2014;10:745-8. [PubMed]
- Teusink AC, Hall PD. Toxicities of gemcitabine in patients with severe hepatic dysfunction. Ann Pharmacother 2010;44:750-4. [Crossref] [PubMed]
- Sharma RA, Decatris MP, Santhanam S, et al. Reversibility of liver failure secondary to metastatic breast cancer by vinorelbine and cisplatin chemotherapy. Cancer Chemother Pharmacol 2003;52:367-70. [Crossref] [PubMed]
- Donelli MG, Zucchetti M, Munzone E, et al. Pharmacokinetics of anticancer agents in patients with impaired liver function. Eur J Cancer 1998;34:33-46. [Crossref] [PubMed]
- Poplin E, Roberts J, Tombs M, et al. Leucovorin, 5-fluorouracil, and gemcitabine: a phase I study. Invest New Drugs 1999;17:57-62. [Crossref] [PubMed]
- Berlin JD, Alberti DB, Arzoomanian RZ, et al. A phase I study of gemcitabine, 5-fluorouracil and leucovorin in patients with advanced, recurrent, and/or metastatic solid tumors. Invest New Drugs 1998-1999;16:325-30. [Crossref] [PubMed]
- Hidalgo M, Castellano D, Paz-Ares L, et al. Phase I-II study of gemcitabine and fluorouracil as a continuous infusion in patients with pancreatic cancer. J Clin Oncol 1999;17:585-92. [Crossref] [PubMed]
- Vincenzi B, Imperatori M, Picardi A, et al. Liver toxicity in colorectal cancer patients treated with first-line FOLFIRI-containing regimen: a single institution experience. Expert Rev Anticancer Ther 2015;15:971-6. [Crossref] [PubMed]
- Miller AA, Murry DJ, Owzar K, et al. Phase I and pharmacokinetic study of sorafenib in patients with hepatic or renal dysfunction: CALGB 60301. J Clin Oncol 2009;27:1800-5. [Crossref] [PubMed]
- Geschwind JF, Kudo M, Marrero JA, et al. TACE Treatment in Patients with Sorafenib-treated Unresectable Hepatocellular Carcinoma in Clinical Practice: Final Analysis of GIDEON. Radiology 2016;279:630-40. [Crossref] [PubMed]
- Geschwind JF, Gholam PM, Goldenberg A, et al. Use of Transarterial Chemoembolization (TACE) and Sorafenib in Patients with Unresectable Hepatocellular Carcinoma: US Regional Analysis of the GIDEON Registry. Liver Cancer 2016;5:37-46. [Crossref] [PubMed]


