Carbohydrate antigen 19-9 (CA 19-9) is an independent prognostic indicator in pseudomyxoma peritonei post cytoreductive surgery and perioperative intraperitoneal chemotherapy
Introduction
Pseudomyxoma peritonei (PMP) is a clinical syndrome characterized by gradual accumulation of mucin in the peritoneal space, most often as a consequence of invasion or rupture of a low-grade appendiceal mucinous neoplasm into the peritoneum. Historically PMP was treated with repeated surgical debulking and/or systemic chemotherapy. It is usually considered a relatively indolent disease when able to be managed with serial debulking, however patients ultimately become inoperable and succumb to cachexia and intestinal obstruction secondary to the massive accumulation of mucin known as ‘jelly belly’. The past two decades has observed a paradigm shift in the management of this disease with multimodal treatment involving cytoreductive surgery (CRS) and perioperative intraperitoneal chemotherapy (PIC) becoming the new standard of care. Compared with serial debulking, CRS and PIC results in superior long-term survival and in many circumstances is definitive, curative therapy (1-3).
A number of histological classifications of PMP exist (4-6). The most commonly referenced classification is that of Ronnett et al., who described two types of PMP – disseminated peritoneal adenomucinosis (DPAM) and peritoneal mucinous carcinomatosis (PMCA), each with its distinct prognostic implications (4). The former carries a long-term survival of up to 85% post-CRS and PIC; the latter much less favorable (1-4). However, even within the DPAM variant, there is a considerable disparity in outcomes that is not well described by the literature.
We have previously reported on the possible influence of pre-operative serum carbohydrate antigen 19-9 (CA 19-9) on survival in this disease (7). In this article we aim to further explore the value of CA 19-9, along with carcinoembryonic antigen (CEA) and carbohydrate antigen 125 (CA-125) in further stratifying survival in a larger cohort on patients. We present important knowledge on the significance of CA 19-9 overexpression in DPAM, and to a lesser extent PMCA. This is of relevance to treatment rationalization (patient selection, prioritization for surgery and trial of adjuvant chemotherapy) and opens avenues for novel treatment in this disease.
Patients and methods
Patients
Informed consent from patients and institutional review board approval was obtained for data storage in the prospective surgical database.
Clinicopathologic data of patients who underwent CRS and PIC from Jan 1996 to Mar 2012 at St George Hospital (Sydney, Australia) were retrieved from a prospective database. A retrospective chart review was undertaken to obtain treatment and follow-up data. Histopathology and pre-operative serum tumor markers were confirmed from the computerized hospital system. All patients were followed until Mar 2012 or until death.
Serum CA 19-9, CA-125 and CEA were measured at a median of 1 day prior to surgery. All marker levels were performed at the Sutherland Centre for Immunology laboratory. Serum CA-125 levels were measured with the Cobas Elecsys CA 125 II assay, CA 19-9 with the Cobas Elecsys 19-9 assay and CEA with the Cobas Elecsys CEA assay, all manufactured by Roche. All assays were performed using manufacturer’s instructions on the Modular Analytics E170 immunoassay analyzer. A CA-125 level of >35 U/L, CA 19-9 of >40 U/mL and CEA of >3 ng/mL were considered positive or elevated outside the laboratory reference range.
PMP was classified into disseminated peritoneal adenomucinosis (DPAM), peritoneal mucinous carcinomatosis (PMCA) or peritoneal mucinous carcinomatosis with intermediate or discordant features (PMCA-I/D) according to Ronnett’s criteria (4).
Operative management
All patients were treated in a uniform fashion; CRS was undertaken with intent of removing all macroscopic intraperitoneal tumor deposits according to Sugarbaker’s technique (8). Briefly, a midline laparotomy from xiphoid to pubis was performed to gain abdominal exposure. This is followed by an exploration to characterize the volume of disease (see below). One to six peritonectomy procedures may be required: (I) greater omentectomy-splenectomy; (II) left upper quadrant peritonectomy; (III) right upper quadrant peritonectomy; (IV) lesser omentectomy-cholecystectomy with stripping of the omental bursa; (V) pelvic peritonectomy with sleeve resection of the sigmoid colon; and/or (VI) antrectomy.
The size and distribution of tumor nodules were determined intraoperatively using the Peritoneal Cancer Index (PCI) as described by Jacquet and Sugarbaker (9). The abdomen is divided into nine regions and the small bowel into four: each assigned a lesion-size (LS) score of 0 to 3, which would be representative of the largest implant visualized. LS-0 denotes the absence of implants, LS-1 indicates implants <0.25 cm, LS-2 between 0.25 and 5 cm, and LS-3 >5 cm or a confluence of disease. These figures amount to a final numerical score of 0-39. The amount of residual disease was quantified using the completeness of cytoreduction (CC) score. A CC-0 score indicates that there is no visible residual disease. A CC-1 score denotes that the remaining tumor nodules are less than 0.25 cm. A CC-2 score indicates tumor nodules between 0.25 and 2.5 cm. A CC-3 score indicates tumor nodules greater than 2.5 cm or a confluence of disease.
The PIC protocol consisted of heated intraperitoneal chemotherapy (HIPEC) and early postoperative intraperitoneal chemotherapy (EPIC). HIPEC was administered via the open abdominal technique to eradicate residual microscopic disease. The chemotherapeutic agent used was mitomycin-C (10-12.5 mg/m2) in 3 litres of 1.5% dextrose peritoneal dialysis solution at 42 °C for 90 minutes. Patients with suspected high-grade disease have been treated with bi-directional chemotherapy since 2010. These patients received oxaliplatin 350 mg/m2 IP and 5-fluorouracil 400 mg/m2 IV plus folinic acid 50 mg IV 1 hour prior to HIPEC. EPIC with 5-flurouracil (650 mg/m2) in 1 L of 1.5% dextrose peritoneal dialysis solution was administered. EPIC was delivered once stable postoperatively and was scheduled for a total of five days. This was withheld in patients who were at high risk of developing postoperative complications (e.g., multiple anastomoses) or had already developed early post-operative complications.
Statistical analysis
Correlation between CA 19-9 levels and PCI was studied using the Pearson’s Correlation test. The relationship between CA 19-9 subgroups with histopathology and CC-score was examined with the Student’s t-test.
Overall survival (OS) was defined as time from day of CRS to death. The impact of clinicopathologic and treatment variables on OS as guided by the literature was calculated using the Kaplan Meier method. Survival distributions were compared for significance using the log-rank test.
Variables deemed significant by univariate analysis were entered into multivariate analysis using the Cox proportional hazards regression model for adjustment.
Missing data was handled with the pairwise deletion approach; i.e. a variable may be unavailable for one patient but the case was still included in the analysis of other variables. It was not anticipated that this would be statistically problematic, as missing tumor markers were largely from the same patients.
A P-value of <0.05 was considered significant for all analyses. All statistical analyses were conducted by SPSS® for Windows version 20.0 (SPSS, Munich, Germany).
Results
Over a 16-year period, 224 patients were treated with CRS and PIC at our institution. Detailed histopathological reports and follow-up data were not available for 6 patients; they were excluded from this study. The median follow-up period was 19 months (range, 0-177, SD=29).
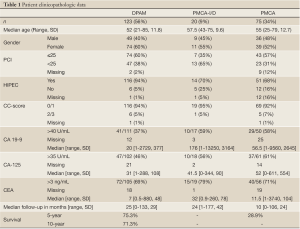
The patient clinicopathologic data is summarized in Table 1.
Full table
Survival outcomes for all histopathological subtypes
Baseline CA 19-9, CEA and CA-125 were available for 178, 181 and 180 patients respectively. Median survival for the entire cohort was 102 months.
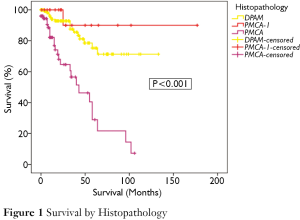
Figure 1 demonstrates the overall survival for the entire cohort stratified by histopathological subtypes. There was a significant difference in survival between the groups (P<0.001). 75% of patients with DPAM were projected to survive to 5 years and 71% to 10 years (median survival not reached). In the PMCA group, 29% were alive at 5-year, with a median survival of 43 months. In the PMCA-I/D group, 5- and 10-year survivals were 90% and 90% respectively (median survival not reached).
Patients who were CA 19-9 negative had a better survival than those who were seropositive. The 5-year survivals were 90% and 46% respectively (P<0.001, Figure 2A). There was no significant difference in survival between patients based on CEA or CA-125 positivity, P=0.116 and P=0.128 respectively.
The impact of CA 19-9 on survival was further delineated when the cohort was split into 4 subgroups: CA 19-9 ≤40 U/mL, 41-100 U/mL, 101-1,000 U/mL and >1,000 U/mL to determine if the absolute level of CA 19-9 was of consequence. 90% of patients with CA 19-9 ≤40 U/mL were alive at 5 years. Patients with CA 19-9 ranging between 41-100 U/mL and 101-1,000 U/mL had a 5-year survival of 67% and 54% respectively. In contrast, the 5-year survival of patients with CA 19-9 >1,000 U/mL was 12%. (P<0.001, Figure 2B).
In these 4 subgroups, CA 19-9 levels were found to be associated with histopathological subtypes (P=0.033) and PCI (P=0.025, r=0.170). There was no significant relationship between CA 19-9 and CC-score (P=0.126).
Survival outcomes for DPAM and PMCA-I/D subtypes
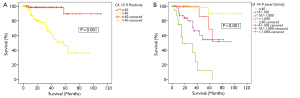
There was a disparity in survival between patients who were CA 19-9 positive and those in the normal range. 5-year survivals for CA 19-9 negative and CA 19-9 positive patients were 90% and 58% respectively (P<0.001, Figure 3A).
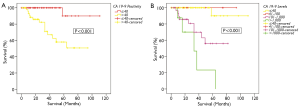
This group was then further split into 4 subgroups as above; CA 19-9 ≤40 U/mL, 40-100 U/mL, 100-1,000 U/mL and >1,000 U/mL. In patients with CA 19-9 >1,000 U/mL, the actuarial 5-year survival was 23%. This was in contrast to patients with CA 19-9 ≤100 U/mL, where the 5-year survival was more than 90% (P<0.001, Figure 3B).
100% of CEA-negative patients survived at 5 years, as opposed to 73% of CEA positive patients. The difference was not statistically significant (P=0.062). CA-125 positivity had no significant impact on survival (P=0.233).
Other variables found to have an adverse effect on overall survival in the univariate analyses were CC-score 2/3 (P<0.001), PCI >25 (P<0.001) and male gender (P=0.017).
Results from the Cox regression model are displayed in Table 2. Only CA 19-9 positivity was found to be an independent prognostic factor for poor survival (P=0.034)
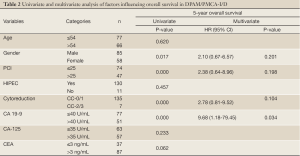
Full table
Survival outcomes for PMCA subtype
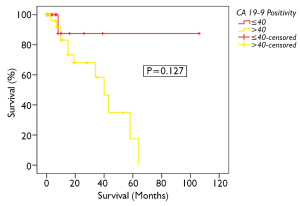
Overall 5-year survival for CA 19-9 positive and negative patients was 18% and 88% respectively, however this was not statistically significant (Figure 4, P=0.127). This group could not be further divided as done above due to the smaller number of patients. CA-125 and CEA were not found to significantly impact on survival in PMCA patients (P=0.373 and 0.368 respectively, Table 3).On univariate analysis, the only factor found to be significantly associated with survival was CC-score (P<0.001, Table 3).
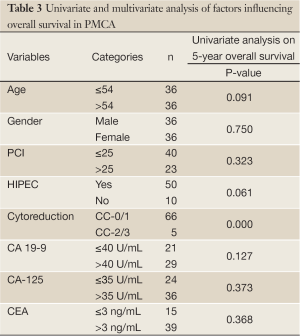
Full table
A multivariate analysis was not performed in this group.
Discussion
There are a number of patient, pathologic and treatment related variables that influence post-cytoreductive outcomes in PMP. Perhaps the most important known prognostic determinant is tumor histopathology; the DPAM subtype behaves in a substantially more favorable manner than PMCA (2,4). However, even within the DPAM group, there is a considerable variability in outcomes. We aim to examine the impact of pre-operative tumor markers in further stratifying survival.
Whilst several authors have suggested the clinical utility of baseline tumor markers in PMP, the papers have not distinguished between the 2 histopathological groups, which is the principal finding of the current study. It was difficult to compare the studies due to inconsistent end-points. In a large cohort of 532 patients by the Sugarbaker group, CEA and CA 19-9 were both found to correlate significantly with survival (P<0.001 and P=0.008 respectively) on univariate analyses (10). Baratti et al. and van Ruth et al. described the association of CA 19-9 positivity with increased risk of recurrence but had no significant impact on survival (11,12). The Basingstoke group described CEA as a predictor of recurrence in 35 patients (P=0.003). The 2-year recurrence free interval was 53% in patients with elevated CEA compared to 94% in patients with normal CEA (13). Ross et al. found that CA-125 elevation was associated with reduced survival in disseminated appendiceal malignancies (14). Chua et al. published that elevated baseline tumor markers including CA 19-9 increases the likelihood of developing early recurrence post definitive cytoreduction in the DPAM and PMCA-I/D subtypes and that this in turn leads to significantly reduced survival (15). The same authors also identified CA 19-9 as an independent factor contributing to reduced progression-free survival in patients with appendiceal peritoneal carcinomatosis (16). Additionally, tumor markers were incorporated into a scoring system by Caskin et al., along with histopathology and haematological status, to predict short term survival (<12 months) and identify patients who may not benefit from CRS (17).
CA 19-9, also known as Sialyl-Lewisa (sLea), was first described in 1979 by Koprowski, using a monoclonal antibody produced by immunizing a mouse with a colorectal carcinoma cell line (18). It is a carbohydrate antigen expressed (primarily as a glycolipid) on the surface of some epithelial tumors of the gastrointestinal tract. The overexpression of CA 19-9 has been associated with neoplastic progression (19). It is a known ligand for E-selectin, an endothelial leucocyte adhesion molecule (20) and has been hypothesized to increase the metastatic potential of some malignancies.
For instance, several studies have successfully used monoclonal antibodies directed against sLea to inhibit its binding with E-selectin, resulting in anti-tumor activity and inhibition of metastasis (21,22).
Serum CA 19-9 level provides prognostic information in gastrointestinal and hepatobiliary cancers. It is established that elevated serum CA 19-9 in patients with colorectal carcinoma is one of the most powerful prognostic indicators for earlier recurrence and mortality (23,24). It is also strongly associated with the presence and/or development of hepatic metastasis (25). CA 19-9 positivity also predicts stage and survival in patients with pancreatic adenocarcinoma (26).
The baseline diagnostic sensitivity of CA 19-9, CA-125 and CEA in the current study was comparable to the literature. The relatively low sensitivity of CA 19-9 may be partly attributed to the fact that the antigenic determinant of CA 19-9 is a sialylated derivative of the Lewisa blood group antigen; genotypically, Lewisa-b- individuals (about 5-10% of the general population) cannot synthesize the CA 19-9 antigen (27).
We chose to split our cohort into 2 separate histopathological subtypes for analysis – there is a strong case in doing so given that they are so prognostically distinct from one another. The PMCA-I/D subtype was grouped with DPAM, as, it has less of a propensity for lymphatic and haematogenous dissemination like PMCA. CA 19-9 was found to be an independent prognostic indicator in DPAM (Figure 3, Table 2) but the association was not as statistically robust in PMCA. As demonstrated in Figure 4, there was an observable difference between the survival curves, but it is likely that the log-rank test was limited by numbers (CA 19-9 ≤40 U/mL n=21, CA 19-9 >40 U/mL n=29).
In addition to tumor marker positivity, the absolute marker level was also found to be prognostically significant (Figure 2B,3B). The distribution of CA 19-9 values was positively skewed. Only a small proportion of patients in the DPAM and PMCA-I/D group supersecrete CA 19-9 (i.e. >1,000 U/mL), but these patients behave in a similar pattern as patients with PMCA. The 5-year survival of DPAM and PMCA-I/D patients with CA 19-9 >1,000 U/mL was 20%, which is comparable to 29% of the PMCA group regardless of marker level.
Our results suggest that CA 19-9 is a strong prognostic indicator in PMP. There is convincing evidence in our investigation that supports this in the DPAM/PMCA/I-D subgroup, and with further interrogation may be applicable in PMCA as well. One of the difficulties of this investigation was in choosing a cut-off point for what is considered significant. As aforementioned, the distribution of CA 19-9 is skewed to the right; many patients might only have a slightly elevated marker level, which may not be clinically meaningful. In analyzing the DPAM/PMCA-I/D group, we chose to split the group rather arbitrarily based on degree of marker elevation (≤40 U/mL, 40-100 U/mL, >100 U/mL and >1,000 U/mL). We found that arbitrary grouping or splitting into quartiles all resulted in demonstrating the same pattern of behavior, however, our method allows for setting a practical cut-off that can be utilized in clinical practice or further research protocols. More specifically, we believe that CA 19-9 >100 U/mL may be a more appropriate discriminator than marker positivity itself and given the findings, should be incorporated as part of a staging scheme for PMP. This could potentially identify patients with DPAM/PMCA-ID who may benefit from adjuvant chemotherapy, which is not currently standard practice.
Using CA 19-9 as part of a staging system may also provide some objectivity in dealing with tumors classified as PMCA-I/D. In most studies, patients with PMCA-I/D tend to exhibit a similar biological behavior to that of frank PMCA (1,4). However, in the current cohort, the PMCA-I/D group appeared to portend a survival time similar to the DPAM group. Presently the histopathological critieria defining this group of patients remains complex and insufficient attention has been given in the pathological analysis. As Bradley et al. have described from their institutional experience, the bi-grouping of PMP may be appropriate as in our context, given the similar outcomes of both PMCA-I/D and DPAM (5). Ronnett’s criteria defines PMCA-I/D as peritoneal lesions that contain predominantly features of DPAM but with focal areas of well-differentiated mucinous adenocarcinoma or discordant features. Use of the word ‘predominantly’ thus leaves room for ambiguity as there are no specific cut-offs per se. Due to this ambiguity, our institution recognizes that there are limitations in relying solely on Ronnett's criteria in determining prognosis. Perhaps the treatment decision should be more heavily based on the pre-operative CA 19-9 level, given that even in patients with PMCA, 88% of CA 19-9 negative patients survive to 5-year.
Our study suggests an intimate relationship between CA 19-9 and PMP which should be further scrutinized, not only for the purpose of developing novel treatments but more pertinently, rationalizing current treatment strategies. As some authors have done for other gastrointestinal malignancies, the potential role of CA 19-9 in mediating tumor cell adhesion and disease progression in PMP merits investigation in a laboratory setting to deepen our understanding of the disease’s inherent biological behavior. If a true relationship exists, CA 19-9/sLea may be a conceivable target for immunotherapy in this disease. Our results also provide relevant prognostic information for the DPAM subtype for the purpose of staging and prioritizing urgency of surgery, as even in patients with apparently indolent disease, survival outcomes vary widely. In addition, elevated CA 19-9 may be useful in identifying patients who would potentially benefit from adjuvant therapy and/or closer post-operative surveillance.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Sugarbaker PH. New standard of care for appendiceal epithelial neoplasms and pseudomyxoma peritonei syndrome. Lancet Oncol 2006;7:69-76.
- Baratti D, Kusamura S, Nonaka D, et al. Pseudomyxoma peritonei: biological features are the dominant prognostic determinants after complete cytoreduction and hyperthermic intraperitoneal chemotherapy. Ann Surg 2009;249:243-9.
- Chua TC, Moran BJ, Sugarbaker PH, et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol 2012;30:2449-56.
- Ronnett BM, Zahn CM, Kurman RJ, et al. Disseminated peritoneal adenomucinosis and peritoneal mucinous carcinomatosis. A clinicopathologic analysis of 109 cases with emphasis on distinguishing pathologic features, site of origin, prognosis, and relationship to “pseudomyxoma peritonei”. Am J Surg Pathol 1995;19:1390-408.
- Bradley RF, Stewart JH 4th, Russell GB, et al. Pseudomyxoma peritonei of appendiceal origin: a clinicopathologic analysis of 101 patients uniformly treated at a single institution, with literature review. Am J Surg Pathol 2006;30:551-9.
- Misdraji J, YantissRK, Graeme-Cook FM, et al. Appendiceal mucinous neoplasms: a clinicopathologic analysis of 107 cases. Am J Surg Pathol 2003;27:1089-103.
- Chua TC, Chong CH, Liauw W, et al. Inflammatory markers in blood and serum tumor markers predict survival in patients with epithelial appendiceal neoplasms undergoing surgical cytoreduction and intraperitoneal chemotherapy. Ann Surg 2012;256:342-9.
- Sugarbaker PH. Peritonectomy procedures. Ann Surg 1995;221:29-42.
- Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. In: Sugarbaker PH, eds. Peritoneal Carcinomatosis: Principles Of Management. Boston, MA: Kluwer Academic Publishers, 1996;359-74.
- Carmignani CP, Hampton R, Sugarbaker CE, et al. Utility of CEA and CA 19-9 tumor markers in diagnosis and prognostic assesment of mucinous epithelial cancer of the appendix. J Surg Oncol 2004;87:162-6.
- Baratti D, Kusamura S, Martinetti A, et al. Prognostic value of circulating tumor markers in patients with pseudomyxoma peritonei treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol 2007;14:2300-8.
- van Ruth S, Hart AA, Bonfrer JM, et al. Prognostic value of baseline and serial carcinoembryonic antigen and carbohydrate antigen 19-9 measurements in patients with pseudomyxoma peritonei treated with cytoreduction and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol 2002;9:961-7.
- Alexander-Sefre F, Chandrakumaran K, Banerjee S, et al. Elevated tumor markers prior to complete tumor removal in patients with pseudomyxoma peritonei predict early recurrence. Colorectal Dis 2005;7:382-6.
- Ross A, Sardi A, Nieroda C, et al. Clinical utility of elevated tumor markers in patients with disseminated appendiceal malignancies treated by cytoreductive surgery and HIPEC. Eur J Surg Oncol 2010;36:772-6.
- Chua TC, Liauw W, Morris DL. Early recurrence of pseudomyxoma peritonei following treatment failure of cytoreductive surgery and perioperative intraperitoneal chemotherapy is indicative of a poor survival outcome. Int J Colorectal Dis 2012;27:381-9.
- Chua TC, Al-Alem I, Saxena A, et al. Surgical cytoreductionand survival in appendiceal cancer peritoneal carcinomatosis: an evaluation of 46 consecutive patients. Ann Surg Oncol 2011;18:1540-6.
- Cashin PH, Graf W, Nygren P, et al. Patient selection for cytoreductive surgery in colorectal peritoneal carcinomatosis using serum tumor markers: an observational cohort study. Ann Surg 2012;256:1078-83.
- Koprowski H, Steplewski Z, Mitchell K, et al. Colorectal carcinoma antigen detected by hybridoma antibodies. Somatic Cell Genet 1979;5:957-71.
- Gong E, Hirobashi S, Shimosato Y. Expression of carbohydrate antigen 19-9 and stage-specific embryonic antigen in non-tumorous and tumorous epithelia of the human colon and rectum. J Natl Cancer Inst 1985;75:447-54.
- Takada A, Ohmori K, Yoneda T, et al. Contribution of carbohydrate antigens sialyl Lewis A and sialyl Lewis X to adhesion of human cancer cells to vascular endothelium. Cancer Res 1993;53:354-61.
- Kawamura YI, Kawashima R, Fukunaga R, et al. Introduction of Sd(a) carbohydrate antigen in gastrointestinal cancer cells eliminates selectin ligands and inhibits metastasis. Cancer Res 2005;65:6220-7.
- Sawada R, Sun SM, Wu X, et al. Human monoclonal antibodies to sialyl-Lewis (CA 19-9) with potent CDC, ADCC, and antitumor activity. Clin Cancer Res 2011;17:1024-32.
- Nakayama T, Watanabe M, Teramoto T, et al. CA19-9 as a predictor of recurrence in patients with colorectal cancer. J Surg Oncol 1997;66:238-43.
- Wang WS, Lin JK, Chiou TJ, et al. CA19-9 as the most significant prognostic indicator of metastatic colorectal cancer. Hepatogastroenterology 2002;49:160-4.
- Isozaki H, Ohyama T, Mabuchi H. Expression of cell adhesion molecule CD44 and sialyl Lewis A in gastric carcinoma and colorectal carcinoma in association with hepatic metastasis. Int J Oncol 1998;13:935-42.
- Ferrone CR, Finkelstein DM, Thayer SP, et al. Perioperative CA19-9 levels can predict stage and survival in patients with resectable pancreatic adenocarcinoma. J Clin Oncol 2006;24:2897-902.
- Kannagi R. Carbohydrate antigen sialyl Lewis a--its pathophysiological significance and induction mechanism in cancer progression. Chang Gung Med J 2007;30:189-209.