
Surgery, radiotherapy, chemotherapy, targeted therapy, and immunotherapy of rectal adenocarcinoma with penile metastasis: a case report
Highlight box
Key findings
• In addition to surgery, radiotherapy, and chemotherapy, strategic therapies including targeted therapy and immunotherapy may provide a more comprehensive and effective choice for the treatment of rectal cancer, especially for advanced rectal cancer.
What is known and what is new?
• What is known is that previous studies including local excision, partial or complete penectomy, external beam radiotherapy, brachytherapy and chemotherapy have been more palliative than curative. These studies have demonstrated a poor prognosis in advanced rectal cancer.
• What is new is that our patient insisted on positive treatment based on systemic therapy combined with immunotherapy and survived for up to 4 years and 6 months following penectomy, despite multiple rectal cancer metastases. Strategic therapies including surgery, radiotherapy, chemotherapy, targeted therapy, and immunotherapy may contribute to improving the quality of life of patients and prolonging their survival.
What is the implication, and what should change now?
• Patients with advanced rectal cancer including penile metastasis may derive more benefit from comprehensive treatment.
Introduction
The penis is an uncommon site of metastatic colorectal adenocarcinoma (1). The first reported case of penile metastases from rectal adenocarcinoma was reported by Eberth in 1870 (2). Beatriz Nunes et al. reported a patient with a metastasis to the glans penis from a rectal adenocarcinoma underwent palliative treatment with radiotherapy and chemotherapy, remaining asymptomatic and disease-free at one year follow-up (3). Boubacar Efared et al. disclosed a patient with penile metastasis from rectal adenocarcinoma who accepted his adjuvant chemotherapy (XELOX regimen, oxaliplatin plus capecitabine) (4). A patient underwent palliative chemotherapy treatment and still alive 4 months after diagnosis of penile metastases (5). Lee et al. reported a patient with metastatic carcinoma from the rectal cancer and the patient was still alive after receiving palliative chemotherapy with modified FOLFOX-6 (mFOLFOX-6; oxaliplatin with 5-fluorouracil and folinic acid) plus bevacizumab (6). On average, penile metastasis from rectal adenocarcinoma can occur within 2 years after the diagnosis of the primary tumor (7).
Several palliative or curative methods have been applied in previous cases, such as chemotherapy, total penectomy, and radiotherapy; however, the patient prognosis remains poor. The knowledge gaps and limitations of prior study is low incidence of rectal adenocarcinoma with penile metastasis, inexperience of treatment and the lack of standard treatment guidelines. The clinical significance of this study is early detection, precise diagnosis, patient’s positive psychology that we guided and comprehensive treatment including surgery, radiotherapy, chemotherapy, targeted therapy, and immunotherapy.
We applied targeted therapy and immunotherapy in this case, and the prognosis was not poor after phallectomy. We also performed a literature search and found 56 previously reported cases of rectal cancer spreading to the penis (Table 1) and the table revealed the ages, interval of metastasis, treatment of rectal cancer, treatment of metastasis, other metastasis and prognosis of patients from different regions or countries. The prognosis of our patient is 54 months after being diagnosed with metastasis of penile cancer, and the patient benefited from the strategic treatment and improved the quality of life. We present the following article in accordance with the CARE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-84/rc).
Table 1
| Author, ref | Year | Age, years | Interval of metastasis, months | Treatment of rectal cancer |
Treatment of MTS | Other metastatic sites | Prognosis |
|---|---|---|---|---|---|---|---|
| Our case | 2022 | 59 | 54 | APR | TP, PC, RT, cetuximab, right inguinal lymphadenectomy bevacizumab, sintilimab injection | Lymphonodus, bone, lung | 54 m |
| Lee (6) | 2020 | 74 | 9 | Neoadjuvant CRT, APR | PC | None | 4 m |
| Marghich (5) | 2019 | 47 | 4 | APR | PC | Bone, lung, lymphonodus | 4 m |
| Ahmad (8) | 2019 | 51 | NA | NA | PC | NA | NA |
| Kuliavas (9) | 2018 | 41 | 17 | CTR, PP, RT | TP | Bladder | 2 m |
| Efared (4) | 2017 | 46 | 8 | APR | TP | None | NA |
| Kozan (10) | 2016 | 58 | 18 | APR | TP | None | Follow-up |
| Nunes (3) | 2015 | 66 | 15 | RT | CRT, RT | None | Follow-up |
| Christodoulidou (11) | 2015 | 70 | 24 | Neoadjuvant CRT, LAR | TP | Lung | NA |
| Alzayed (12) | 2015 | 70 | 26 | Neoadjuvant CRT/RT, APR | NA | Lung | NA |
| Persec (7) | 2014 | 43 | 24 | LAR, neoadjuvant CRT | PP | NA | 6 m |
| Hajianfar (13) | 2014 | 78 | 24 | LAR, neoadjuvant CRT | PP | NA | 3 m |
| McGuinness (14) | 2013 | 61 | 60 | LAR | Circumcision, glansectomy | NA | NA |
| Dorsett (1) | 2012 | 61 | NA | CRT | Penectomy | Lung | 4 m |
| Kimura (15) | 2012 | 57 | 16 | APR | Penectomy | NA | 24 m, alive |
| Gbenou (16) | 2011 | 79 | 24 | LAR | Refused | NA | 6 m |
| Seo (17) | 2011 | 72 | 7 | APR | CRT | Liver, bone | Death |
| Yildirim (18) | 2010 | 78 | 36 | Neoadjuvant CRT/RT, APR | CRT | Diffused | Poor |
| Küronya (19) | 2009 | 65 | 54 | LAR | CPT | None | NA |
| Park (20) | 2009 | 43 | 24 | APR | CPT | Lung, bone lymphonodus | NA |
| Chung (21) | 2008 | 69 | Meanwhile | NA | RT | Liver | 6 m, alive |
| Ketata (22) | 2007 | 59 | 312 | APR | CPT | Lung | 16 m, alive |
| Murhekar (23) | 2007 | 78 | 24 | APR | Refused | NA | 4 m |
| Pellicé i Vilalta (24) | 2006 | NA | NA | NA | NA | NA | NA |
| Cherian (25) | 2006 | 75 | 60 | APR | PT | Lung | 12 m |
| Cathomas (26) | 2006 | 58 | 26 | LAR | CRT | Liver, lung | Poor |
| Appu (27) | 2006 | 65 | 24 | APR | RT | None | 12m |
| Yilmaz (28) | 2004 | 71 | 24 | LAR | NA | None | 2 w, death |
| Lo (29) | 2004 | 56 | 24 | APR | NA | NA | NA |
| Tan (30) | 2002 | 53 | Meanwhile | APR | RT | NA | NA |
| Sukumar (31) | 2001 | 75 | 2 | APR, CTR | RT | NA | 2 m |
| Al-Mashat (32) | 2000 | 65 | 19 | APR | No treatment | NA | 5 m |
| Lange (33) | 1997 | 42 | NA | APR | Surgical treatment | NA | NA |
| Cuvillier (34) | 1995 | NA | 29 | APR | CRT | NA | 15 m |
| Comandone (35) | 1992 | NA | NA | NA | PT | NA | NA |
| Doré (36) | 1989 | 58 | NA | NA | NA | NA | NA |
| Mukamel (37) | 1987 | 58 | 2 | NA | No treatment | NA | 5 m |
| Khubchandani (38) | 1986 | 71 | 48 | APR | RT | NA | 9 m |
| Honda (39) | 1985 | 69 | 10 | CTR, RT | Surgical treatment | NA | NA |
| Honda (39) | 1985 | 60 | 24 | APR | NA | NA | NA |
| Okumura (40) | 1984 | 45 | 22 | Resection | NA | NA | NA |
| Kakehi (41) | 1984 | 72 | NA | NA | NA | NA | NA |
| Kakehi (41) | 1984 | 42 | NA | NA | NA | NA | NA |
| Kakehi (41) | 1984 | 66 | NA | NA | NA | NA | NA |
| Zanetti (42) | 1983 | NA | NA | NA | NA | NA | NA |
| Kumar (43) | 1980 | 70 | 4 | APR | Fluorouracil and irradiation | Lung | 5 d |
| Rees (44) | 1975 | 41 | 33 | APR | Penectomy | NA | 8 m, alive |
| Rees (44) | 1975 | 71 | 24 | APR | CRT | Lymphonodus | Good |
| Sakkas (45) | 1974 | 48 | NA | CRT | CRT | NA | 6 m |
| Bachrach (46) | 1973 | 59 | NA | NA | NA | liver | 2 w |
| Poser (47) | 1972 | 59 | NA | APR | NA | NA | 2 m, alive |
| Tagart (48) | 1967 | 75 | 108 | APR | TP | None | 5 y |
| Ongenae (49) | 1967 | NA | NA | NA | NA | NA | NA |
| Oehlschlaegel (50) | 1966 | NA | NA | NA | NA | NA | NA |
| Cattell (51) | 1951 | 30 | 29 | APR | Resection | NA | NA |
| Eberth (2) | 1870 | 40 | NA | NA | NA | NA | NA |
MTS, metastasis; APR, abdominoperineal resection; LAR, low anterior resection; TP, total penectomy; PP, partial penectomy; PC, palliative chemotherapy; RT, radiotherapy; CRT, chemoradiotherapy; LAR, low anterior resection; CTR, chemotherapy; NA, not available; d, days; m, months; y, years.
Case presentation
Four years and six months ago, a 54-year-old Chinese man presented with penile pain and dysuria for 6 months, without a family history of rectal or penile cancers. The patient was hospitalized, and the physical examination showed multiple hard nodules of the penile shaft and glans with visible skin ulceration on the penile glans (Figure 1). The man died at the age of 59 years due to multiple organ failure. He had undergone abdominoperineal resection for adenocarcinoma of the rectum 7 years and 6 months previously, and the contrast-enhanced computed tomography (CT) scan showed that this case was consistent with the postoperative manifestations of rectal cancer. Given that the penile metastasis was solitary, we performed a total penectomy and paracentetic suprapubic cystostomy 4 years and 6 months prior.
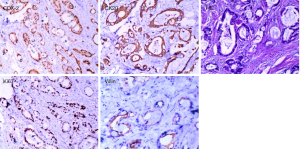
Pathological biopsy revealed multiple metastatic adenocarcinoma tissues in the penis (Figure 2), and immunohistochemistry confirmed the hypothesis of rectal adenocarcinoma metastasis. Tumor cells were positive for CDX-2 (caudal type homeobox transcription factor 2), Villin, CK20 (cytokeratin20) and the Ki-67 index reached up to 50%; negative for TTF-1 (thyroid transcription factor1), and CK7 (cytokeratin7).
The patient received chemotherapy and radiotherapy after surgery, but metastasis to the right regional nodes was found 23 months after penectomy. Genetic testing was performed after the patient completed right inguinal lymphadenectomy. The patient accepted therapy involving surgery, palliative chemotherapy, radiotherapy, right inguinal lymphadenectomy, Cetuximab, Bevacizumab, and Sintilimab injection.
Six cycles of chemotherapy were administered with Iritecan 320 mg d1 and Tessio 60 mg d1−d14 53 months previously, and radiotherapy with DT 59.6 Gy/32 f was administered 47 months previously. Right inguinal lymph node metastasis was found 31 months ago, and right inguinal lymphadenectomy was performed. Six cycles of therapy with Cetuximab 800 mg d1, Iritecan 320 mg d2 and continuously-pumped Fluorouracil 3.5 g was used 29 months previously. Pelvic bone and lymph node metastases were found 24 months ago, radiotherapy with DT 50 Gy/25 f was administered 20 months previously, and the patient was then treated with cetuximab.
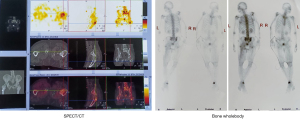
The PET-CT found multiple bone (right femur, right sacroiliac joint, ischia, pubis) metastases, and the gene test results showed that Bevacizumab was sensitive 15 months previously. The patient also felt severe low back pain and need painkillers. Bevacizumab 400 mg d0, irinotecan 300 mg d1, and fluorouracil 0.5 g d2–6 were administered 14 months previously. Cindilimab 200 mg was also used 13 months previously. Meanwhile, the pelvic bone and lymph node metastases were treated by radiotherapy with DT 50 Gy/25 f 12 months previously. Moreover, the lymph nodes, soft tissue, and femur tumor metastases were treated by radiotherapy with DT 60 Gy/30 f 12 months previously. One cycle of Cindilimab, Bevacizumab, and Capecitabine was applied 10 months prior.
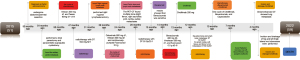
Radiation necrosis, hip soft tissue infection, and pyogenic buttocks were found 7 months previously, and the patient tended to lay prone and could not lie on his back (Figure 3). We disinfect with iodophor (the available iodine content, 4.4 to 5.0 g/L) twice a day if there are cutaneous manifestations of metastatic disease to the penis. Chest-enhanced CT showed lung metastasis and Cindilimab was administered 4 months previously. Incision and drainage of hip and left thigh lateral root abscesses were also performed 4 months previously (Figures 4-6). We added the Figure 6 as a timeline figure to arrange the events and interventions in chronological order with dates.
All procedures performed in the study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s family member for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Herein, we reported the outcomes of our experience treating a patient with rectal adenocarcinoma metastasis to the penis and found that the patient, who was treated with active treatments, had positive control outcomes. In addition, we observed that the patient may have derived more benefits in terms of disease control from strategic therapies.
Colorectal cancer has become common worldwide and is a frequent cause of cancer-related death (52). Surgical treatment is an effective therapy for rectal tumors. It is reported that lymphadenectomy with venation is preferred for radical resection in some patients (53). Rectal cancer patients have more symptoms and decreased performance status in the presence of advanced disease, and the quality of life of these patients can also be impacted (54). Penile cancer is a rare malignancy, and patients with this condition tend to delay seeking medical attention, which may lead to poor outcomes (55). Recent findings in penile cancer have motivated several trials evaluating new modalities of systemic treatments, especially immunotherapy (56). Sintilimab is a recombinant humanized lgG4 anti-PD-1 antibody, with affinity to human programmed cell death protein 1 (PD-1). Lv et al. (57) reported that a patient of recurrent and metastatic penile squamous cell carcinoma obtained progression-free survival with continuous sintilimab. Immune-based treatments coupled with systemic therapy may offer benefits to patients with advanced penile cancer (58).
Tumor response was investigator assessed according to the Response Evaluation Criteria in Solid Tumors (RECIST; version 1.1) (59) or immune Response Evaluation Criteria in Solid Tumors (iRECIST) (60). Cancers develop in complex tissue environments, depending upon for sustained growth, invasion and metastasis. Cancers develop in complex tissue environments, depending upon for sustained growth, invasion and metastasis. The establishment of an antitumor immune response and memory can eliminate tumor cells, prevent recurrence in the primary region and inhibit metastasis to distant sites (61). Cytokine therapy (62), cancer vaccines (63), immune checkpoint blockade (ICB) (64) and adoptive cell transfer (ACT) therapy (65) focus on boosting immune response and immune cell activities in the tumor microenvironment (TME), and the ICB function can inhibit local tumor progression and systemic metastasis.
Immunotherapies have led to substantial changes in cancer treatment and been a popular topic in cancer research. Mismatch repair deficiency or microsatellite instability-high is significantly associated with long-term immunotherapy-related responses and better prognosis in colorectal and noncolorectal malignancies treated with immune checkpoint inhibitors (66). The efficacy of PD-1/PD-L1 inhibitors varies among different classic driver oncogene mutations (67), KRAS-mutant patients appeared to respond better to anti-PD-1/PD-L1 immunotherapy, while patients with EGFR mutant disease may obtain benefits from immunotherapies, but the detailed mechanistic explanation needs further research. As a specific RNA adaptor, THUMPD1 (68) is significantly associated with immune cell infiltration, tumor mutational burden (TMB), microsatellite instability (MSI), immune checkpoints and neoantigen. Patients with higher THUMPD1 expression exhibited a better prognosis in kidney renal clear cell carcinoma (KIRC) and rectum adenocarcinoma (READ), while worse prognosis in liver hepatocellular carcinoma (LIHC) patients, so THUMPD1 may be a novel predictor to evaluate cancer prognosis and immune therapy efficacy in diverse cancer types. More predictors are required further exploration and research.
Penile metastases from rectal cancer are extremely rare. The most common primary malignancies with penis metastases are urogenital cancers (69%); gastrointestinal origin accounts for 19%, with the sigmoid colon and rectum accounting for 12% of all such cases (69). Although rectal adenocarcinoma is a common malignant tumor of the digestive system, which usually metastasizes to local lymph nodes, the lung, the liver, and bone, penile metastasis from rectal cancer is rare, and these lesions can occur without any liver or lung involvement (16). Perineal pain, induration, urethral obstruction, priapism, and hematuria are the most common associated symptoms. In 40% of patients, priapism is considered a prominent feature. Metastases can present as plaques, wart-like nodules, ulceration, erythema, or induration of the penis (5). The mass ordinarily progresses to involve the corpora cavernosa with extension into the neighboring perineal subcutaneous tissue, the corpus spongiosum, and bulb (6). Although pain is not the patient’s initial symptom, it is often the most prominent symptom in the later stage. Given that penile metastasis from rectal adenocarcinoma can commonly occur within 2 years after the diagnosis of a primary tumor, combining the history and other clinical manifestations would help to suspect the disease early. To confirm the diagnosis of penile metastasis, immunohistochemical staining is beneficial for discriminating the origin of the cancer. Primary adenocarcinoma of the colon and metastatic lesions can be strongly negative for CK 7 and positive for CK 20 (17). The patient received chemotherapy for metastatic disease until disease progression and unacceptable toxicity and he died with progressive disease, and tissue tumor markers, such as AFP, CEA, and HCG can be used for differential diagnosis (18).
Despite its rich and interconnected vasculature and its proximity to the genitourinary system, in which the prostate and bladder are both popular sites for tumor metastasis, metastatic tumors from colorectal cancer rarely involve the penis. There have been many hypotheses regarding penile metastasis from rectal cancer. Yet, the retrograde venous route is the most commonly accepted route of metastasis since the dorsal venous system of the penis can communicate with the venous plexus system of the pelvis. Through this mechanism, the retrograde lymphatic course may be how tumor cells from pelvic organs (rectosigmoid, prostate, urinary bladder) reach the corpus cavernosal and the glans via the lymphatics and iliac inguinal nodes. Other less common ways include direct extension, arterial spread, or the iatrogenic spread by instruments, as well as secondary penile root tumors from adjacent pelvic organs (4). Since the left and right inguinal lymph nodes are fibrous adipose tissue and no cancer was observed in our case, we speculated that our case was via the retrograde venous metastasis route.
Various therapies have been attempted for the treatment of penile metastases, such as total penectomy, radiotherapy, and chemotherapy. However, these approaches have been more palliative than curative. Overall survival normally varies from 7 months to 2 years when the tumor spreads diffusely. Long-term survival (9 years) has been observed following aggressive surgery (penile amputation), with the best patient outcomes occurring in cases of penile metastasis being the only evident region of recurrence. More clinical treatment research exploration, multi-center study and public network database to achieve global sharing of cancer data may bring greater clinical benefits to the patients. More comprehensive analysis of multiple markers may also provide the appropriate strategy in the future.
The choice of treatment of our patient have a palliative intention, but these therapeutic options may lead to superior treatment outcomes especially when considering the patient's positive attitude. We speculate that comprehensive treatment including surgery, radiotherapy, chemotherapy, targeted therapy, and immunotherapy and helping patients have a positive and optimistic attitude may contribute to improve the clinical management of similar cases.
Conclusions
The metastatic prognosis remains poor regardless of the treatment options, except for lesions where metastasis is only limited to the penis. The metastatic carcinoma of the penis in our case was solitary, and surgical penectomy seemed to improve the prognosis, which was not poor. We found that the patient may derive more benefit from strategic therapies and positive attitude. Thus, the similar patients may benefit from multiple aggressive therapies and survive longer.
Acknowledgments
Funding: This work was supported by the Traditional Chinese Medicine Science and Technology Project of Shandong Provincial Health Commission (No. 2021Q133).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-84/rc
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-84/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-84/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in the study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s family member for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dorsett F, Hou J, Shapiro O. Metastasis to the penis from rectal adenocarcinoma. Anticancer Res 2012;32:1717-9. [PubMed]
- Eberth CJ. Krebsmetastasen des corpus cavernosum penis. Archiv für pathologische Anatomie und Physiologie und für klinische Medicin 1870;51:145-6.
- Nunes B, Matias M, Alves A, et al. Metastasis to the Glans Penis: An Unusual Site of Rectal Cancer Recurrence. Acta Med Port 2015;28:525-7. [Crossref] [PubMed]
- Efared B, Ebang GA, Tahirou S, et al. Penile metastasis from rectal adenocarcinoma: a case report. BMC Res Notes 2017;10:564. [Crossref] [PubMed]
- Marghich O, Dkhissi Y, Alila M, et al. Penile metastases of rectal adenocarcinoma after abdominoperineal resection: a case report. J Med Case Rep 2019;13:233. [Crossref] [PubMed]
- Lee TG, Son SM, Kim MJ, et al. Penile metastasis in rectal cancer with pathologic complete response after neoadjuvant chemoradiotherapy: The first case report and literature review. Medicine (Baltimore) 2020;99:e21215. [Crossref] [PubMed]
- Persec Z, Persec J, Sovic T, et al. Penile metastases of rectal adenocarcinoma. J Visc Surg 2014;151:53-5. [Crossref] [PubMed]
- Ahmad SW, Daze RP, Arvaneh S, et al. Painful Penile Plaques: A Rare Case Report of Rectal Adenocarcinoma with Cutaneous Metastasis to the Penis. Cureus 2019;11:e5095. [Crossref] [PubMed]
- Kuliavas J, Dulskas A, Drachneris J, et al. Penile Metastasis from Rectal Carcinoma: Case Report and Review of the Literature. Visc Med 2018;34:389-92. [Crossref] [PubMed]
- Kozan AA, Smith AM, Ilsley DW, et al. First case of penile metastasis following abdominoperineal resection with VRAM flap reconstruction. J Surg Case Rep 2016;2016:rjw182. [Crossref] [PubMed]
- Christodoulidou M, Sahdev V, Muneer A, et al. A rare case of metachronous penile and urethral metastases from a rectal mucinous adenocarcinoma. BMJ Case Rep 2015;2015:bcr2015212706. [Crossref] [PubMed]
- Alzayed MF, Artho G, Nahal A, et al. Penile metastasis from rectal cancer by PET/CT. Clin Nucl Med 2015;40:e245-50. [Crossref] [PubMed]
- Hajianfar R, Muniesa M, Gomez E, et al. Metastasis of rectal adenocarcinoma to the penis. Arch Esp Urol 2014;67:353-6. [PubMed]
- McGuinness LA, Floyd MS Jr, Lucky M, et al. Penile metastases treated with partial glansectomy and adjuvant radiotherapy 5 years after an initial diagnosis of rectal cancer. BMJ Case Rep 2013;2013:bcr2013200829. [Crossref] [PubMed]
- Kimura Y, Shida D, Nasu K, et al. Metachronous penile metastasis from rectal cancer after total pelvic exenteration. World J Gastroenterol 2012;18:5476-8. [Crossref] [PubMed]
- Goris Gbenou MC, Wahidy T, Llinares K, et al. Atypical phimosis secondary to a preputial metastasis from rectal carcinoma. Case Rep Oncol 2011;4:542-6. [Crossref] [PubMed]
- Seo HS, Kim ES, Kim S, et al. A Case of Urethral Metastasis from Sigmoid Colon Cancer Diagnostically and Prognostically Indicated by F-18 FDG PET/CT. Nucl Med Mol Imaging 2011;45:319-23. [Crossref] [PubMed]
- Yildirim M, Coskun A, Pürten M, et al. A clinical case of the penile metastasis from the rectal carcinoma. Radiol Oncol 2010;44:121-3. [Crossref] [PubMed]
- Küronya Z, Bodrogi I, Lövey J, et al. Metachronous metastasis from rectal adenocarcinoma to the penis--case report. Magy Onkol 2009;53:263-6. [PubMed]
- Park JC, Lee WH, Kang MK, et al. Priapism secondary to penile metastasis of rectal cancer. World J Gastroenterol 2009;15:4209-11. [Crossref] [PubMed]
- Chung TS, Chang HJ, Kim DY, et al. Synchronous penile metastasis from a rectal carcinoma. Int J Colorectal Dis 2008;23:333-4. [Crossref] [PubMed]
- Ketata S, Boulaire JL, Soulimane B, et al. Metachronous metastasis to the penis from a rectal adenocarcinoma. Clin Colorectal Cancer 2007;6:657-9. [Crossref] [PubMed]
- Murhekar KM, Majhi U, Mahajan V, et al. Penile metastasis from rectal carcinoma. Indian J Cancer 2007;44:155-6. [Crossref] [PubMed]
- Pellicé i Vilalta C. Subcutaneous prepuce metastasis secondary to rectal adenocarcinoma. Arch Esp Urol 2006;59:926-author reply 7. [PubMed]
- Cherian J, Rajan S, Thwaini A, et al. Secondary penile tumours revisited. Int Semin Surg Oncol 2006;3:33. [Crossref] [PubMed]
- Cathomas R, Geldart TR, Iveson T, et al. An unusual differential diagnosis of penile warts: metastases from rectal carcinoma. Int J STD AIDS 2006;17:491-2. [Crossref] [PubMed]
- Appu S, Lawrentschuk N, Russell JM, et al. Metachronous metastasis to the penis from carcinoma of the rectum. Int J Urol 2006;13:659-61. [Crossref] [PubMed]
- Yilmaz E, Batislam E, Altinok G, et al. Isolated late penile metastasis of an adenocarcinoma of the rectum. Urol Int 2004;72:261-3. [Crossref] [PubMed]
- Lo S, Crew J. Penile metastasis from rectal carcinoma. Singapore Med J 2004;45:299. [PubMed]
- Tan BK, Nyam DC, Ho YH. Carcinoma of the rectum with a single penile metastasis. Singapore Med J 2002;43:39-40. [PubMed]
- Sukumar N, Qureshi A. Adenocarcinoma of rectum metastasizing to penis. Med J Malaysia 2001;56:255-6. [PubMed]
- Al-Mashat F, Sibiany A, Rakha S, et al. Penile metastasis from rectal carcinoma. Saudi Med J 2000;21:379-81. [PubMed]
- Lange G, Fagot H, Faulques B, et al. Penile metastasis of recto-sigmoid adenocarcinoma. Apropos of a case. Ann Chir 1997;51:294-6. [PubMed]
- Cuvillier X, Donnaint A, Rigot JM, et al. Report of a case of penile metastasis. Review of the literature. Prog Urol 1995;5:1009-11. [PubMed]
- Comandone A, Bau G, Mo A, et al. Metastasis to the penis from carcinoma of the rectum. A clinical case. Minerva Gastroenterol Dietol 1992;38:49-52. [PubMed]
- Doré B, Grange P, Aubert J. Penile metastasis disclosing cancer of the rectum. Apropos of a case. Ann Urol (Paris) 1989;23:158-60. [PubMed]
- Mukamel E, Farrer J, Smith RB, et al. Metastatic carcinoma to penis: when is total penectomy indicated? Urology 1987;29:15-8. [Crossref] [PubMed]
- Khubchandani M. Metachronous metastasis to the penis from carcinoma of the rectum. Report of a case. Dis Colon Rectum 1986;29:52-4. [Crossref] [PubMed]
- Honda M, Kameoka H, Miyoshi S, et al. Secondary penile tumors: report of two cases. Hinyokika Kiyo 1985;31:2273-9. [PubMed]
- Okumura S, Hirasawa S, Yui Y, et al. A clinical case of secondary tumor of the penis from the rectum, with malignant priapism. Hinyokika Kiyo 1984;30:205-15. [PubMed]
- Kakehi Y, Arai E, Katamura E, et al. Metastatic penile tumors: report of three cases. Hinyokika Kiyo 1984;30:363-9. [PubMed]
- Zanetti PP, Calabrò B, Gagna G, et al. Metastasis to the corpus cavernosum of the penis from a rectal cancer. Minerva Urol 1983;35:69-71. [PubMed]
- Kumar PP, Newland JR. Metastatic carcinoma of the penis. J Natl Med Assoc 1980;72:55-8. [PubMed]
- Rees BI. Secondary involvement of the penis by rectal cancer. Br J Surg 1975;62:77-9. [Crossref] [PubMed]
- Sakkas JL, Mandrekas A, Androulakis J, et al. Urologic complications in malignant disease of the rectosigmoid colon. South Med J 1974;67:287-91. [Crossref] [PubMed]
- Bachrach P, Dahlen CP. Metastatic tumors to the penis. Urology 1973;1:359-62. [Crossref] [PubMed]
- Poser H, Kuttig H. Penile metastasis of rectal adenocarcinoma. Ther Ggw 1972;111:976-81. [PubMed]
- Tagart RE. Secondary deposit of rectal carcinoma in the penis. Proc R Soc Med 1967;60:501. [Crossref] [PubMed]
- Ongenae D, Lagae J. Metastatic carcinoma of the penis. Arch Belg Dermatol Syphiligr 1967;23:403-6. [PubMed]
- Oehlschlaegel G. The corpora cavernosa penis as a site for secondary tumor seeding in cancer of the rectum. Z Haut Geschlechtskr 1966;41:425-9. [PubMed]
- CATTELL RB. MACE AJ. Metastasis to the penis from carcinoma of the rectum. J Am Med Assoc 1951;146:1230-1. [Crossref] [PubMed]
- Vendrely V, Rivin Del Campo E, Modesto A, et al. Rectal cancer radiotherapy. Cancer Radiother 2022;26:272-8. [Crossref] [PubMed]
- Ge W, Gong HY, Shao LH, et al. Lymphadenectomy with venation is preferred compared to skeletonization for patients with rectal and sigmoid colon cancer: a retrospective cohort study. J Gastrointest Oncol 2022;13:1746-52. [Crossref] [PubMed]
- Cihan YB, Ozturk A. The effect of demographic, clinical, and pathological data on quality of life in rectum cancer. Support Care Cancer 2021;29:7411-20. [Crossref] [PubMed]
- Engelsgjerd JS, LaGrange CA. Penile Cancer. 2023. Copyright © 2022, StatPearls Publishing LLC.; 2022.
- Alencar AM Jr, Sonpavde G. Emerging Therapies in Penile Cancer. Front Oncol 2022;12:910335. [Crossref] [PubMed]
- Lv C, Wu C, Zhang Y, et al. Sintilimab-Induced Diabetic Ketoacidosis in a Patient with Radiation and Multichemorefractory Penile Cancer: A Case Report and Literature Review. Curr Oncol 2022;29:7987-93. [Crossref] [PubMed]
- Joshi VB, Spiess PE, Necchi A, et al. Immune-based therapies in penile cancer. Nat Rev Urol 2022;19:457-74. [Crossref] [PubMed]
- Schwartz LH, Litière S, de Vries E, et al. RECIST 1.1-Update and clarification: From the RECIST committee. Eur J Cancer 2016;62:132-7. [Crossref] [PubMed]
- Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 2017;18:e143-52. [Crossref] [PubMed]
- Cha JH, Chan LC, Song MS, et al. New Approaches on Cancer Immunotherapy. Cold Spring Harb Perspect Med 2020;10:a036863. [Crossref] [PubMed]
- Sockolosky JT, Trotta E, Parisi G, et al. Selective targeting of engineered T cells using orthogonal IL-2 cytokine-receptor complexes. Science 2018;359:1037-42. [Crossref] [PubMed]
- Hollingsworth RE, Jansen K. Turning the corner on therapeutic cancer vaccines. NPJ Vaccines 2019;4:7. [Crossref] [PubMed]
- Poggio M, Hu T, Pai CC, et al. Suppression of Exosomal PD-L1 Induces Systemic Anti-tumor Immunity and Memory. Cell 2019;177:414-427.e13. [Crossref] [PubMed]
- O'Donnell JS, Teng MWL, Smyth MJ. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat Rev Clin Oncol 2019;16:151-67. [Crossref] [PubMed]
- Zhao P, Li L, Jiang X, et al. Mismatch repair deficiency/microsatellite instability-high as a predictor for anti-PD-1/PD-L1 immunotherapy efficacy. J Hematol Oncol 2019;12:54. [Crossref] [PubMed]
- Jin R, Liu C, Zheng S, et al. Molecular heterogeneity of anti-PD-1/PD-L1 immunotherapy efficacy is correlated with tumor immune microenvironment in East Asian patients with non-small cell lung cancer. Cancer Biol Med 2020;17:768-81. [Crossref] [PubMed]
- Li K, Liu J, Yang X, et al. Pan-cancer analysis of N4-acetylcytidine adaptor THUMPD1 as a predictor for prognosis and immunotherapy. Biosci Rep 2021;41:BSR20212300. [Crossref] [PubMed]
- Cocci A, Hakenberg OW, Cai T, et al. Prognosis of men with penile metastasis and malignant priapism: a systematic review. Oncotarget 2018;9:2923-30. [Crossref] [PubMed]
(English Language Editor: A. Kassem)





