
Short-term clinical effects and inflammatory response of natural orifice specimen extraction surgery versus conventional laparoscopic-assisted surgery for the treatment of sigmoid and rectal cancer
Highlight box
Key findings
• Natural orifice specimen extraction surgery effectively decreases the duration for the resumption to semi-liquid diet, and length of postoperative hospital stay. Furthermore, it also has fewer postoperative incision infections and causes significantly less impairment of the immune system compared to conventional laparoscopic-assisted surgery.
What is known and what is new?
• Natural orifice specimen extraction surgery has the advantages of smaller wounds and less invasiveness but its clinical benefits in the treatment of sigmoid and rectal cancer still need to be evaluated.
• This study investigated the postoperative clinical outcomes and inflammatory response of natural orifice specimen extraction surgery versus conventional laparoscopic-assisted surgery in the treatment of sigmoid and rectal cancer.
What are the implications, and what should change now?
• Natural orifice specimen extraction surgery is superior to conventional laparoscopic-assisted surgery in the treatment of sigmoid and rectal cancer because it not only has clinically significant benefits but also has fewer adverse effects on the immune system.
Introduction
At present, surgery is the main treatment for colorectal cancer. Compared to the open surgery, the laparoscopic surgical approach has been broadly used owing to its advantages in terms of rapid recovery, less intraoperative blood loss and postoperative complications (1). The conventional laparoscopic-assisted radical resection of colorectal cancer is required an auxiliary incision in the abdominal wall for anastomosis and reconstruction of the digestive tract. With the improvement of minimally invasive technology, the emergence of NOSES provides a new method for the treatment of colorectal cancer, including sigmoid and rectal cancer. This operation utilizes a laparoscopic approach for intracorporeal anastomosis without auxiliary incision, by completing the reconstruction of the digestive tract in vivo, which has the advantages of smaller wounds and less invasiveness (2,3). However, the clinical outcomes and benefits of NOSES in sigmoid and rectal cancer have not been fully evaluated. It has been hypothesized that the immunologic response might be related to the surgical outcomes, as the postoperative immune response not only responds to postoperative infection but also to tumor spread and metastases (4-6). This retrospective study was conducted to investigate the short-term clinical benefits of NOSES versus conventional laparoscopic-assisted surgery for the treatment of sigmoid and rectal cancer. The postoperative clinical outcomes and inflammatory responses were used to reflect the short-term clinical effects and the potential recovery ability, respectively. We present the following article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-144/rc).
Methods
Participants
A total of 112 patients with sigmoid and rectal cancer admitted to Affiliated Hospital of Guangdong Medical University from February 2019 to April 2022 were included, with 60 cases in the observation group and 52 cases in the control group. All participants underwent a series of evaluations. The inclusion criteria were as follows: (I) tumors located in sigmoid or upper rectum, and those less than 5 cm, as determined by preoperative radiologic examination; (II) patients diagnosed with colorectal cancer according to the pathological criteria; (III) cases consistent the indications for surgical treatment and without contraindications; (IV) non-emergency surgery; and (V) without perforation, bleeding, obstruction pelvic infection, or anorectal disease.
Patients were excluded based on the following criteria: (I) serious dysfunction of the heart, kidneys, or other organs; (II) multiple primary tumors; (III) imaging evidence of local invasion and metastatic cancer; (IV) previous surgical treatment of colorectal cancer; (V) estimated survival time <6 months; (VI) history of abdominal, pelvic, and anorectal surgery; (VII) patients with autoimmune or infectious diseases and (VIII) have preoperative steroid use. This study conformed to the provisions of the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the ethics committee of the Affiliated Hospital of Guangdong Medical University (Registration No. PJ2020-090) and informed consent was taken from all the patients.
Surgical procedure
The surgical procedure was performed under general anesthesia in a sterilized operating area and with sterile clothing. A five-hole method was employed to place the abdominal wall puncture devices. Tumor dissociation and dissection of lymphoid tissue were conducted according to the laparoscopic radical sigmoidectomy or proctectomy protocol. Cefuroxime was routinely given 30 minutes before the operation and the first day after surgery. Re-dosing given was required if the operation duration exceeds 3 hours.
The control group was treated with conventional laparoscopic-assisted radical sigmoidectomy or proctectomy. A plastic wound protector was used to cover the abdominal incision. After separation of the mesocolon, the proximal colon was split and placed the anvil. Then tumor tissue was removed.
The observation group was treated with CRC-NOSES VI or V. A linear cutter stapler was utilized to split proximal and distal colon of tumor. A vaginal or rectal incision was made after lavage, and then a plastic wound protector was used to put the anvil into enterocoelia and remove the excised diseased tissue. Next, the anvil was introduced into the proximal colon. The open rectal or vaginal stump was closed with a linear stapler.
In the two groups, the circular stapling device was introduced into the rectum, and an end-to-end anastomosis was performed. Postoperative interventions such as anti-infection, fluid rehydration, and fasting were then carried out (Figure 1).
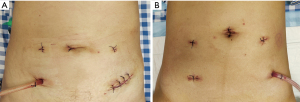
Outcomes measures
(I) Perioperative recovery evaluation (7): outcomes such as operation time, intraoperative blood loss (which was evaluated by the gauze sponges), duration for the first postoperative exhaust, duration for the first postoperative defecation, duration for resumption of a semi-liquid diet, length of hospital stay, and postoperative complications were included in the evaluation. (II) T lymphocyte subsets indicators (8), including CD3+, CD4+, CD8+, and CD4+/CD8+ were observed. Next, 5 mL of fasting peripheral venous blood was extracted, detected using an EPICSXL flow cytometer (BECKMAN COULTER, USA), and the CD4+/CD8+ ratio was calculated. (III) Immunoglobulin levels (9), including immunoglobulin G, M, and A (IgG, IgM, and IgA) were also observed. 5 mL of fasting venous blood was extracted, placed at room temperature for 1 h, and then centrifuged to separate serum. Subsequently, immune turbidimetry detection was utilized in the analysis (4). C-reactive protein (CRP) was detected by radioimmunoassay, and interleukin (IL)-6 and tumor necrosis factor (TNF)-α were detected by enzyme-linked immunosorbent assay (10). All peripheral venous blood samples were obtained 1 day preoperatively and 3 days postoperatively.
Statistical methods
Statistical analysis and graphs were performed and generated using R version 3.6.2. [R Core Team (2022). R Foundation for Statistical Computing, Vienna, Austria]. Quantitative variables were analyzed using the Student’s t-test and were expressed as the mean ± standard deviation (SD). Categorical variables were expressed as a percentage (%) and were contrasted by using Pearson’s Chi-Square (χ2) test or Fisher’s exact test as appropriate. P<0.05 was considered to indicate statistical significance.
Results
The clinical characteristic of the participants
The observation group comprised a total of 35 males and 25 females, with 59.75±11.82 years. Also, there were 32 males and 20 females in the control group, with 62.77±11.38 years. The differences in the clinical characteristics between observation and control groups, including age, gender, BMI, duration, and distribution of tumor, tumor size, node, metastasis (TNM) stages were not statistically significant (P>0.05, Table 1).
Table 1
| Clinical characteristics | Control group (n=52) | Observation group (n=60) | t/χ2 | P |
|---|---|---|---|---|
| Age (years), mean ± SD | 62.77±11.38 | 59.75±11.82 | 1.37 | 0.173 |
| Gender, n (%) | 0.12 | 0.730 | ||
| Male | 32 (61.54) | 35 (58.33) | ||
| Female | 20 (38.46) | 25 (41.67) | ||
| BMI (kg/m2), mean ± SD | 23.05±2.86 | 23.35±2.30 | 0.63 | 0.520 |
| Tumor size (mm), mean ± SD | 27.90 ± 5.82 | 28.89 ± 5.43 | 0.92 | 0.360 |
| pTNM stages, n (%) | 1.55 | 0.908 | ||
| I | 20 (38.46) | 24 (40.00) | ||
| IIA | 11 (21.15) | 14 (23.33) | ||
| IIB | 4 (7.69) | 2 (3.33) | ||
| IIIA | 3 (5.77) | 2 (3.33) | ||
| IIIB | 9 (17.31) | 12 (20.00) | ||
| IIIC | 5 (9.62) | 6 (10.00) |
SD, standard deviation; BMI, body mass index; TNM, tumor, node, metastasis staging.
Perioperative outcomes
Compared to the conventional laparoscopic-assisted surgery, significant differences were observed in the effect of NOSES on the duration for the operation time (t=2.83, P=0.006), duration for the resumption of a semi-liquid diet (t=2.17, P=0.032), length of postoperative hospital stay (t=2.74, P=0.007), and postoperative incision infection (χ2=7.32, P=0.009). However, the differences between the groups in terms of the perioperative outcomes of intraoperative blood loss (t=1.26, P=0.209), duration for the first postoperative exhaust (t=1.73, P=0.086), duration for the first postoperative defecation (t = 0.99, P=0.320), and postoperative complications including anastomotic leakage (χ2=0.02, P=0.884) are not significant, as shown in Table 2.
Table 2
| Perioperative outcomes | Control group | Observation group | t/χ2 | P |
|---|---|---|---|---|
| Operation time (min) | 165.40±49.58 | 195.72±62.10 | 2.83 | 0.006 |
| Intraoperative blood loss (mL) | 42.12±19.84 | 47.67±25.74 | 1.26 | 0.209 |
| Duration for the first postoperative exhaust (days) | 2.85±1.59 | 2.33±1.57 | 1.73 | 0.086 |
| Duration for the first postoperative defecation (days) | 3.83±1.89 | 3.48±1.84 | 0.99 | 0.320 |
| Duration for resumption of a semi-liquid diet (days) | 4.86±2.05 | 4.02±2.07 | 2.17 | 0.032 |
| Length of postoperative stay in hospital (days) | 9.89±4.48 | 7.80±3.61 | 2.74 | 0.007 |
| Postoperative complications | ||||
| Incision infection | 6 (11.54) | 0 (0.00) | 7.32 | 0.009 |
| Anastomotic leakage | 2 (3.85) | 2 (3.33) | 0.02 | 0.884 |
Data are shown as mean ± SD or n (%).
Postoperative inflammatory response
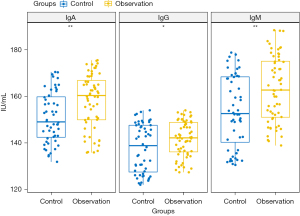
No remarkable differences were observed in the T lymphocyte subset indicators, including CD3+ (t=0.74, P=0.463), CD4+ (t=0.12, P=0.908), CD8+ (t=0.06, P=0.950), and CD4+/CD8+ ratio (t=0.39, P=0.698) between the observation and control group at 3 days postoperatively (as shown in Figure 2, Table 3). The levels of IgG (t=2.29, P=0.024), IgA (t=3.30, P=0.001), and IgM (t=3.38, P=0.001) in the observation group were significantly higher than those in the control group at 3 days after the operation (Figure 3, Table 3). Moreover, the levels of IL-6 (t=4.22, P=5.02E–5), CRP (t=3.73, P=3.5E–4), and TNF-α (t=2.94, P=0.004) in the observation group were significantly lower than those in the control group at 3 days post-surgery (Figure 4, Table 3). There were no significant differences in all indicators between the two clusters preoperatively (P>0.05) (Table 3).

Table 3
| Inflammatory markers | Periods | Control group | Observation group | t | P |
|---|---|---|---|---|---|
| IL-6 (ng/L) | Preop. | 22.35±2.78 | 22.47±3.20 | 0.21 | 0.831 |
| Post. | 43.78±7.85 | 37.92±6.84 | 4.22 | 5.02E–5 | |
| CRP (mg/L) | Preop. | 7.00±0.87 | 7.18±0.89 | 1.07 | 0.289 |
| Post. | 26.34±6.70 | 22.32±4.22 | 3.73 | 3.5E–4 | |
| TNF-α (ng/L) | Preop. | 36.01±1.36 | 36.11±1.62 | 0.37 | 0.709 |
| Post. | 47.25±4.79 | 44.99±3.01 | 2.94 | 0.004 | |
| CD3+ | Preop. | 51.80±2.91 | 51.75±2.96 | 0.08 | 0.936 |
| Post. | 48.02±3.76 | 47.53±3.18 | 0.74 | 0.463 | |
| CD4+ | Preop. | 27.52±3.05 | 26.98±2.93 | 0.97 | 0.336 |
| Post. | 41.87±3.59 | 41.80±2.87 | 0.12 | 0.908 | |
| CD8+ | Preop. | 28.07±1.29 | 28.28±1.35 | 0.82 | 0.416 |
| Post. | 26.49±2.84 | 26.52±2.19 | 0.06 | 0.950 | |
| CD4+/CD8+ | Preop. | 0.98±0.11 | 0.96±0.11 | 1.19 | 0.237 |
| Post. | 1.60±0.24 | 1.59±0.17 | 0.39 | 0.698 | |
| IgG (IU/mL) | Preop. | 139.50±10.03 | 140.25±8.10 | 0.44 | 0.662 |
| Post. | 137.79±10.36 | 141.70±7.65 | 2.29 | 0.024 | |
| IgA (IU/mL) | Preop. | 151.62±3.09 | 152.35±2.74 | 1.32 | 0.190 |
| Post. | 150.87±10.86 | 157.98±11.75 | 3.30 | 0.001 | |
| IgM (IU/mL) | Preop. | 167.87±2.83 | 168.76±2.83 | 1.67 | 0.098 |
| Post. | 153.72±15.33 | 163.12±14.10 | 3.38 | 0.001 |
Data were shown as mean ± standard deviation. IL, Interleukin; CRP, C-reactive protein; TNF, tumor necrosis factor; Ig, immunoglobulin; Preop., preoperative; Post., postoperative.

Discussion
In conventional laparoscopic-assisted surgery, an auxiliary incision in the abdominal wall is required for anastomosis, which will easily cause scarring and also increase the occurrence of complications such as postoperative incision infection (11-14). In NOSES, the resection and separation of tumor tissue and anastomosis are completed intraoperatively in the abdomen. The resected tumor tissue does not need to be taken out through the auxiliary incision but is removed through a natural orifice (anus, vagina, etc.) (15). Compared to conventional laparoscopic-assisted surgery, the greatest advantage of NOSES is that it effectively reduces postoperative scar formation while reducing complications caused by the incision (16-18). Numerous articles have confirmed the advantages of NOSES. In previous studies, specimen extraction by natural orifice and abdominal incision were respectively performed in patients with colorectal cancer, and the reported results demonstrated that the first postoperative exhaust time of NOSES was markedly shorter than that of conventional laparoscopic-assisted surgery, and the postoperative complications were also decreased (19,20). In the present study, we observed notable differences in the duration for the resumption of a semi-liquid diet, length of postoperative hospital stay, and postoperative incision infection between the two groups. In summary, NOSES need a longer operation time, but could effectively decrease the duration for the resumption to semi-liquid diet, and length of postoperative hospital stay. Our results highlighted the benefits of NOSES for the treatment of sigmoid and rectal cancer relative to conventional laparoscopic-assisted radical resection.
Surgery itself is an exogenous stress trauma, which will have a certain impact on the body’s immune function. The damage caused by surgery to the body can also be assessed by detecting the immune function indicators of patients. Moreover, their postoperative recovery can also be reflected by the immune function indicators (21). For instance, T lymphocyte subsets, cellular immunity, and CD4+ activation can release a large number of cytokines that enhance the body’s anti-tumor effect. Furthermore, CD8+ can adhere to and clear viruses (22). Serum immunoglobulin can bind to the tumor antigen and dissolve it. IgG binds macrophages and promotes their phagocytosis to fight cancer. Also, IgA can effectively protect the body mucosa and protect it from damage (23). As an inflammatory factor, CRP is a more sensitive immune indicator in the early stage. In addition, IL-6 is a pro-inflammatory factor that induces inflammatory damage; increased levels of IL-6 are related to the degree of damage and indicate that the body is stimulated or injured externally. TNF-α can be used to assess the degree of trauma by inducing cells to produce various inflammatory cytokines, such as IL-6 (24). In this study, the levels of immunoglobulin indicators were increased, and the levels of inflammatory indicators were decreased in the observation group compared to the control group. This result is consistent with the perioperative clinical outcomes. For example, the shorter duration for resumption of a semi-liquid diet induces faster recovery might be reflected by the immune function indicators. Our results showed a reduced postoperative inflammatory response with the NOSES compared to the conventional laparoscopic-assisted radical resection.
However, it should be noted that NOSES requires the surgeon to operate and master the reconstruction of the digestive tract in vivo. For patients with large tumors or obesity, it is difficult in extraction of specimen from the natural orifice, especially in rectum. Therefore, a comprehensive evaluation is necessary to decide whether the patient meets the criterion of this procedure, which will facilitate the selection of the most appropriate treatment (25). And the comparison for long-term clinical outcomes of two surgical techniques needs to be evaluated in the further studies.
Conclusions
In conclusion, NOSES has clinically relevant advantages, including reducing the duration for the resumption of a semi-liquid diet, the length of postoperative hospital stay, and fewer postoperative incision infections. Moreover, it also causes less impairment of the immune system than conventional laparoscopic-assisted surgery. The findings of the present study support the hypothesis that NOSES can improve the postoperative recovery and has the benefits in reducing the inflammatory response than conventional laparoscopic-assisted surgery in sigmoid and rectal cancer.
Acknowledgments
Funding: This work was supported by Competitive Project of Special Funds for Scientific and Technological development of Zhanjiang City (Grant/Award Numbers: 2021A05080, 2021B01046); Affiliated Hospital of Guangdong Medical University Clinical Research Program (Grant/Award Number: LCYJ2019B007).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-144/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-144/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-144/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-144/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics committee of the Affiliated Hospital of Guangdong Medical University (Registration No. PJ2020-090) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Devoto L, Celentano V, Cohen R, et al. Colorectal cancer surgery in the very elderly patient: a systematic review of laparoscopic versus open colorectal resection. Int J Colorectal Dis 2017;32:1237-42. [Crossref] [PubMed]
- Zhou Z, Chen L, Liu J, et al. Laparoscopic Natural Orifice Specimen Extraction Surgery versus Conventional Surgery in Colorectal Cancer: A Meta-Analysis of Randomized Controlled Trials. Gastroenterol Res Pract 2022;2022:6661651. [Crossref] [PubMed]
- Chin YH, Decruz GM, Ng CH, et al. Colorectal resection via natural orifice specimen extraction versus conventional laparoscopic extraction: a meta-analysis with meta-regression. Tech Coloproctol 2021;25:35-48. [Crossref] [PubMed]
- Mehigan BJ, Hartley JE, Drew PJ, et al. Changes in T cell subsets, interleukin-6 and C-reactive protein after laparoscopic and open colorectal resection for malignancy. Surg Endosc 2001;15:1289-93. [Crossref] [PubMed]
- Tang F, Tie Y, Tu C, Wei X. Surgical trauma-induced immunosuppression in cancer: Recent advances and the potential therapies. Clin Transl Med 2020;10:199-223. [Crossref] [PubMed]
- Guo Q, Shen S, Li X, et al. Inflammatory factors promote the development of colorectal cancer. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2011;36:646-9. [PubMed]
- Park JW, Kang SB, Hao J, et al. Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): 10-year follow-up of an open-label, non-inferiority, randomised controlled trial. Lancet Gastroenterol Hepatol 2021;6:569-77. [Crossref] [PubMed]
- Toor SM, Murshed K, Al-Dhaheri M, et al. Immune Checkpoints in Circulating and Tumor-Infiltrating CD4(+) T Cell Subsets in Colorectal Cancer Patients. Front Immunol 2019;10:2936. [Crossref] [PubMed]
- Roney MSI, Lanagan C, Sheng YH, et al. IgM and IgA augmented autoantibody signatures improve early-stage detection of colorectal cancer prior to nodal and distant spread. Clin Transl Immunology 2021;10:e1330. [Crossref] [PubMed]
- Kamińska J, Kowalska MM, Nowacki MP, et al. CRP, TNF-alpha, IL-1ra, IL-6, IL-8 and IL-10 in blood serum of colorectal cancer patients. Pathol Oncol Res 2000;6:38-41. [Crossref] [PubMed]
- Ikeda A, Fukunaga Y, Akiyoshi T, et al. Wound infection in colorectal cancer resections through a laparoscopic approach: a single-center prospective observational study of over 3000 cases. Discov Oncol 2021;12:2. [Crossref] [PubMed]
- Sheng S, Zhao T, Wang X. Comparison of robot-assisted surgery, laparoscopic-assisted surgery, and open surgery for the treatment of colorectal cancer: A network meta-analysis. Medicine (Baltimore) 2018;97:e11817. [Crossref] [PubMed]
- Biondi A, Grosso G, Mistretta A, et al. Predictors of conversion in laparoscopic-assisted colectomy for colorectal cancer and clinical outcomes. Surg Laparosc Endosc Percutan Tech 2014;24:e21-6. [Crossref] [PubMed]
- Gu C, Wu Q, Zhang X, et al. Single-incision versus conventional multiport laparoscopic surgery for colorectal cancer: a meta-analysis of randomized controlled trials and propensity-score matched studies. Int J Colorectal Dis 2021;36:1407-19. [Crossref] [PubMed]
- Karagul S, Kayaalp C, Sumer F, et al. Success rate of natural orifice specimen extraction after laparoscopic colorectal resections. Tech Coloproctol 2017;21:295-300. [Crossref] [PubMed]
- Izquierdo KM, Unal E, Marks JH. Natural orifice specimen extraction in colorectal surgery: patient selection and perspectives. Clin Exp Gastroenterol 2018;11:265-79. [Crossref] [PubMed]
- Zheng S, Zhao Z, Zheng H, et al. Safety analysis of natural orifice specimen extraction surgery for colorectal cancer. Medicine (Baltimore) 2022;101:e30087. [Crossref] [PubMed]
- Chang SC, Lee TH, Chen YC, et al. Natural orifice versus conventional mini-laparotomy for specimen extraction after reduced-port laparoscopic surgery for colorectal cancer: propensity score-matched comparative study. Surg Endosc 2022;36:155-66. [Crossref] [PubMed]
- Zhang Q, Wang M, Ma D, et al. Short-term and long-term outcomes of natural orifice specimen extraction surgeries (NOSES) in rectal cancer: a comparison study of NOSES and non-NOSES. Ann Transl Med 2022;10:488. [Crossref] [PubMed]
- Ngu J, Wong AS. Transanal natural orifice specimen extraction in colorectal surgery: bacteriological and oncological concerns. ANZ J Surg 2016;86:299-302. [Crossref] [PubMed]
- Karanika S, Karantanos T, Theodoropoulos GE. Immune response after laparoscopic colectomy for cancer: a review. Gastroenterol Rep (Oxf) 2013;1:85-94. [Crossref] [PubMed]
- Ostroumov D, Fekete-Drimusz N, Saborowski M, et al. CD4 and CD8 T lymphocyte interplay in controlling tumor growth. Cell Mol Life Sci 2018;75:689-713. [Crossref] [PubMed]
- Cui M, Huang J, Zhang S, et al. Immunoglobulin Expression in Cancer Cells and Its Critical Roles in Tumorigenesis. Front Immunol 2021;12:613530. [Crossref] [PubMed]
- Milone M, Desiderio A, Velotti N, et al. Surgical stress and metabolic response after totally laparoscopic right colectomy. Sci Rep 2021;11:9652. [Crossref] [PubMed]
- Tang Q, Zhu Y, Xiong H, et al. Natural Orifice Specimen Extraction Surgery versus Conventional Laparoscopic-Assisted Resection in the Treatment of Colorectal Cancer: A Propensity-Score Matching Study. Cancer Manag Res 2021;13:2247-57. [Crossref] [PubMed]
(English Language Editor: A. Kassem)


