
‘CROSS’-ing into the ‘Real World’: a retrospective cohort study of patients receiving trimodality and bimodality therapy for esophageal cancer
Highlight box
Key findings
• We observed worse overall, disease-free, and relapse-free survival among patients receiving bimodality therapy (BMT) in comparison to trimodality therapy (TMT).
• BMT was associated with presenting symptoms, comorbidity, performance status, N-stage, and treatment toxicity.
• Patient choice accounted for 40% of TMT nonadherence.
What is known and what is new?
• Phase III data demonstrate survival benefit for TMT compared to surgery alone; however, BMT is standard treatment for patients with resectable disease who do not undergo esophagectomy.
• We assess a real-world dataset to describe drivers of treatment modality (TMT vs. BMT) and to further contextualize with assessments of survival.
What is the implication, and what should change now?
• Evidenced interventions to mitigate toxicity and to best condition patients for surgery are necessary to optimize patients for esophagectomy.
• Early multidisciplinary consultation and presentation of real-world outcomes to patients may influence adherence in patients who are otherwise fit for surgery.
Introduction
A standard of care for resectable esophageal cancer is trimodality therapy (TMT), consisting of neoadjuvant chemoradiation followed by esophagectomy, with definitive chemoradiation (bimodality therapy, BMT) preferred for patients who would not tolerate surgery, have inoperable disease, or decline surgery (1,2). This standard is evidenced by the phase III ChemoRadiotherapy for Oesophageal cancer followed by Surgery Study (CROSS) trial, which found that, compared to surgery alone, TMT increased overall survival (OS) and progression-free survival (PFS) without negatively impacting health-related quality of life postoperatively (3-5). The smaller Cancer and Leukemia Group B (CALGB) 9781 trial also found OS and PFS benefit with TMT compared to surgery alone but closed early due to poor accrual (6). Survival benefits of TMT have been characterized primarily in literature comparing TMT to surgery alone, and direct comparisons of TMT and BMT are sparse.
Given the demonstrated benefits of surgery, establishing strategies to maximize TMT adherence and address drivers of nonadherence is imperative to bolstering survivorship, while weighing the toxicities of TMT and patient preferences. However, factors driving successful completion of TMT have yet to be fully characterized, especially in patients with classically unfavorable clinical traits and demography, such as those who are older, carry more comorbidities, and have worse performance status. CROSS trial authors did complete a subsequent retrospective (“post-CROSS”) analysis of patients significantly older and more vulnerable than the CROSS trial cohort. Although they found no difference in OS benefit and treatment toxicity compared to the healthier trial cohort, the study excluded patients nonadherent to TMT, which accounted for approximately 16% of patients assessed (7). These data suggest that nonadherence to TMT is both prevalent and not fully predictable at timepoint of initial assessment, and there is little literature to characterize survival for patients who are nonadherent to TMT.
Real world data (RWD) can improve the generalizability of evidence generated through randomized controlled trials (RCTs), especially in clinical practice and community settings (8,9). Here, we present an analysis of RWD from a single-institution retrospective review of patients with clinically resectable esophageal cancer. We aim to explore generalizability of existing evidence for the survival outcomes with TMT noted in CROSS. Utilization of RWD inclusive of patients older and more vulnerable than RCT populations enables the assessment of differences in outcomes between CROSS-eligible and CROSS-ineligible patients based on published criteria. To our knowledge, there are no data comparing outcomes of patients treated with BMT vs. TMT categorized by violation of CROSS exclusion criteria except for one analysis that looked singularly at age (10). Additionally, we aim to characterize and explore documented reasons for nonadherence in our cohort, purposefully including patients nonadherent to TMT as a population of interest, in contrast to the “Post-CROSS” analysis. While previous studies have examined TMT nonadherence in BMT patients, we offer further insight through assessment of clinical factors for association with BMT (vs. TMT) alongside reasons for nonadherence through analysis of a dataset inclusive of both BMT and TMT patients (11,12). We present the following article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-633/rc).
Methods
Patients
We conducted a single institution retrospective review of consecutive patients with non-metastatic surgically resectable esophageal cancer who presented to University of Vermont Medical Center division of Radiation Oncology between 2009–2019. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional review board of the University of Vermont (IRB00000485) and individual consent for this retrospective analysis was waived. Patients were identified from radiation oncology’s MOSAIQ electronic medical record (EMR) system. Patients with biopsy-proven nonmetastatic esophageal adenocarcinoma or squamous cell carcinoma were screened for inclusion in the study.
Patients receiving therapies other than definitive BMT or TMT were excluded. Patients with tumor location in the cervical esophagus were excluded. Patients with Siewert type III tumors (tumor centroid located 2 cm or more below the gastroesophageal junction) were excluded (13). Treatment on a research protocol was not a criterion for exclusion. We did not exclude patients who received a part of their treatment outside our health network, such that patients who received consultation and/or esophagectomy at a higher-volume cancer center were included in our cohort.
Assessment of clinical characteristics
Detailed chart review was conducted using the MOSAIQ and Epic EMR systems. All patients were re-staged utilizing the American Joint Committee on Cancer (AJCC) 8th edition staging manual (14). All patients were analyzed for CROSS trial eligibility based on reported criteria (15). Patients receiving ≥2 chemotherapy treatments prior to chemoradiation were considered to have received induction chemotherapy. For our primary presenting symptom categorical variable, a primary complaint of dysphagia was compared to those presenting with other symptoms including gastrointestinal (GI) bleed/anemia, odynophagia, or asymptomatic incidental discovery. For patients who planned for TMT therapy and did not receive surgery, reasons for nonadherence were recorded based on clear rationale documented in a patient’s medical record. To align with prior studies, categories included personal choice, poor general condition, disease progression, death, unresectable disease on preoperative assessment, and unresectable disease intraoperatively (11,12).
Assessment of efficacy and safety outcomes
For our study, we defined TMT as completed esophagectomy after neoadjuvant chemoradiation. Surgery dates were recorded for all applicable patients, and post-operative mortality within 30 and 90 days from surgery was assessed.
Follow-up
For each patient, the date of most recent follow-up and date of death were recorded as applicable. The reverse Kaplan-Meier (KM) method was used to calculate median follow up (16,17). Patients were assessed for recurrence, which was recorded as the day of clinical evidence of recurrence.
Statistical analysis
Clinical variables were analyzed for association with BMT using univariable logistic regression and multivariable logistic regression. CROSS trial eligibility was excluded from multivariable analysis to prevent collinearity with variables included in criteria. Similarly, age was excluded from multivariable analysis in favor of age-adjusted Charlson Comorbidity Index (CCI), given that these were highly correlated (18).
OS was analyzed using log-rank test and Cox proportional hazards regression, with initial timepoint recorded as the diagnostic biopsy date of the tumor. The proportional hazards assumption was validated graphically by using log-log survival plots. Disease-free survival (DFS) and relapse-free survival (RFS) were generated in the same fashion. Multivariate logistic regression was utilized to assess variables, including modality, for association with OS.
All data analysis was conducted using STATA/SE 16.1 software (Stata, RRID:SCR_012763) of dataset generated as described above (19).
Results
Analysis of clinical variables for association with BMT
Of 186 patients identified from 2009−2019, 95 patients qualified for inclusion, of whom 51 (54%) received TMT. Patient demographics and clinical data at presentation can be found in Table 1. On average, BMT patients had a significantly higher age, higher Eastern Cooperative Oncology Group (ECOG) performance status, and higher age-adjusted CCI. Additionally, a lower proportion of BMT patients met CROSS trial criteria. Tumor location and histology were similar in TMT and BMT patients. There was no significant difference between distance to radiation treatment center and rurality of patient residences between TMT and BMT patients.
Table 1
| Characteristics | TMT, n [%] | BMT, n [%] | P value | |
|---|---|---|---|---|
| Patients included in study | 51 | 44 | – | |
| Age (years), median [IQR] | 63 [58–69.5] | 72.5 [62–79] | 0.007† | |
| Sex | ||||
| Female | 6 [12] | 7 [16] | 0.57 | |
| Male | 45 [88] | 37 [84] | ||
| Weight loss at presentation | ||||
| <5% | 25 [49] | 14 [32] | 0.1 | |
| ≥5% | 26 [51] | 30 [68] | ||
| ECOG performance | ||||
| 0 | 25 [57] | 10 [25] | 0.002 | |
| 1 | 17 [39] | 19 [48] | ||
| 2 | 2 [05] | 11 [28] | ||
| Age-adjusted CCI | ||||
| 2–4 | 23 [45] | 9 [20] | 0.006 | |
| 5–7 | 25 [49] | 24 [55] | ||
| 8–10 | 3 [6] | 11 [25] | ||
| Primary presenting symptom | ||||
| Dysphagia | 39 [76] | 26 [59] | 0.08 | |
| Other | 12 [24] | 18 [41] | ||
| Tumor histology | ||||
| AC | 44 [86] | 36 [82] | 0.58 | |
| SCC | 7 [14] | 8 [18] | ||
| Tumor location | ||||
| Upper/middle | 7 [14] | 9 [20] | 0.42 | |
| Lower/GEJ | 44 [86] | 35 [80] | ||
| Tumor stage (AJCC 8th Ed.) | ||||
| I | 1 [2] | 2 [5] | 0.06 | |
| II | 5 [10] | 5 [12] | ||
| IIB | 7 [14] | 0 [0] | ||
| III | 35 [69] | 30 [70] | ||
| IVA | 3 [6] | 6 [14] | ||
| T-stage | ||||
| cT1 | 1 [2] | 2 [5] | 0.12 | |
| cT2 | 11 [22] | 3 [7] | ||
| cT3 | 39 [76] | 38 [88] | ||
| N-stage | ||||
| cN0 | 28 [55] | 22 [51] | 0.69 | |
| cN1 | 19 [37] | 15 [35] | ||
| cN2-3 | 4 [8] | 6 [14] | ||
| Tumor grade | ||||
| G1 | 4 [8] | 3 [9] | 0.33 | |
| G2 | 29 [60] | 15 [44] | ||
| G3 | 15 [31] | 16 [47] | ||
| History of neoplasm | ||||
| No | 42 [82] | 30 [68] | 0.15 | |
| Yes | 9 [18] | 14 [32] | ||
| Qualify for CROSS trial | ||||
| Eligible | 24 [47] | 3 [7] | <0.001 | |
| Ineligible | 27 [53] | 41 [73] | ||
| Distance from radiation treatment | ||||
| <30 miles | 24 [57] | 24 [55] | 0.54 | |
| ≥30 miles | 27 [53] | 20 [45] | ||
| Rurality by Zip Code (RUCA) | ||||
| Non-rural | 39 [76] | 28 [64] | 0.19 | |
| Rural | 12 [24] | 16 [36] | ||
P values are generated using Fisher’s exact test except †age, which utilized a two-sample t-test. TMT, trimodality therapy; BMT, bimodality therapy; IQR, interquartile range; ECOG, Eastern Cooperative Oncology Group; CCI, Charlson Comorbidity Index; AC, adenocarcinoma; SCC, squamous cell carcinoma; GEJ, gastroesophageal junction; AJCC, American Joint Committee on Cancer; CROSS, ChemoRadiotherapy for Oesophageal cancer followed by Surgery Study; RUCA, rural-urban commuting area codes.
Radiation dose, participation in clinical trial, utilization of induction chemotherapy, and utilization of lodging wraparound services were similar between TMT and BMT patients (see Table 2). Additional characterization of patients receiving induction chemotherapy and chemotherapy agents utilized in all study participants is available in the Tables S1,S2. Only 12% (6/50) of patients receiving TMT received lower-dose radiation therapy (LDRT, dose ≤48.85 Gy10), all receiving the CROSS-regimen dose of 41.4 Gy in 23 fractions. Among BMT patients, 7% (3/43) received LDRT, all of whom did not complete their treatment plan and received 21.33, 28.8, or 41.4 Gy.
Table 2
| Characteristics | TMT, n [%] | BMT, n [%] | P value |
|---|---|---|---|
| Total dose of radiotherapy (Gy), median (IQR) | 50.4 (47.7–50.4) | 50.4 (50.4–50.4) | 0.27† |
| Chemo held during treatment | |||
| No | 25 [68] | 12 [30] | 0.001 |
| Yes | 12 [32] | 28 [70] | |
| Induction chemotherapy | |||
| No | 33 [79] | 33 [89] | 0.24 |
| Yes | 9 [21] | 4 [11] | |
| On clinical trial | |||
| No | 37 [80] | 35 [85] | 0.37 |
| Yes | 9 [20] | 6 [15] | |
| Lodging wraparound service | |||
| Utilized | 27 [56] | 30 [71] | 0.19 |
| Not utilized | 21 [44] | 12 [29] | |
P values are generated using Fisher’s exact test except †radiotherapy dose, which utilized a two-sample t-test. TMT, trimodality therapy; BMT, bimodality therapy; IQR, interquartile range.
A larger proportion of BMT patients had ≥1 chemotherapy cycle held compared to TMT patients (P=0.001). Among BMT patients, 28 (70%) had ≥1 cycle of chemotherapy held. Indication for withholding chemotherapy included cytopenia (15, 54%), poor performance or fatigue (6, 21%), GI toxicity (2, 7%), and other reasons (6, 21%). One patient had multiple documented reasons for holding chemotherapy. Among TMT patients, 12 (32%) patients had ≥1 cycle of chemotherapy held. Indication for withholding chemotherapy included cytopenia (7, 58%), poor performance/fatigue (2, 17%), GI toxicity (1, 8%), and other reasons (2, 17%). There were insufficient data to reliably compare the incidence of individual toxicities between patients receiving BMT and TMT.
On multivariable logistic regression, higher age-adjusted CCI, higher ECOG performance status at presentation, higher N-stage, presentation with a chief complaint other than dysphagia, and held chemotherapy cycles were all significantly associated with BMT (see Table 3).
Table 3
| Variable | Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | ||
| Age at diagnosis | 0.92 | 0.88–0.97 | 0.001 | – | – | – | |
| Not CROSS eligible | 0.08 | 0.02–0.30 | <0.001 | – | – | – | |
| CCI | |||||||
| 5–7 | 0.41 | 0.16–1.06 | 0.07 | 0.18 | 0.042–0.79 | 0.02 | |
| ≥8 | 0.11 | 0.02–0.47 | 0.003 | 0.036 | 0.003–0.38 | 0.006 | |
| ECOG PS at presentation | |||||||
| 1 | 0.36 | 0.13–0.96 | 0.04 | 0.45 | 0.11–1.89 | 0.28 | |
| ≥2 | 0.07 | 0.01–0.39 | 0.002 | 0.12 | 0.015–0.96 | 0.05 | |
| N stage | |||||||
| 1 | 1.34 | 0.44–1.47 | 0.28 | 0.70 | 0.18–2.73 | 0.61 | |
| ≥2 | 0.81 | 0.79–2.30 | 0.48 | 0.023 | 0.001–0.45 | 0.01 | |
| Presentation, no dysphagia | 0.44 | 0.18–1.07 | 0.07 | 0.11 | 0.018–0.74 | 0.02 | |
| Held chemotherapy cycle(s) | 0.21 | 0.08–0.54 | 0.001 | 0.074 | 0.015–0.36 | 0.003 | |
OR greater than one indicates greater odds of trimodality therapy. BMT, bimodality therapy; OR, odds ratio; CI, confidence interval; CROSS, ChemoRadiotherapy for Oesophageal cancer followed by Surgery Study; CCI, Charlson Comorbidity Index; ECOG PS, Eastern Cooperative Oncology Group Performance Status.
TMT adherence
Of patients who ultimately received BMT, only 14 (32%) planned to receive definitive chemoradiation without surgery from the start of treatment. Among patients without planned definitive BMT, we observed 63% adherence to TMT. A large portion of nonadherent patients (40%) declined surgical treatment. Reasons for patient nonadherence to TMT are recorded in Table 4 alongside findings from two similar analyses of nonadherence (11,12). Among the 12 patients who declined surgery, 6 (50%) patients cited preference for non-surgical management, 3 (25%) patients cited concern for morbidity, 2 (17%) patients cited advanced age, and 1 (8%) patient cited optimism based on response to chemoradiation. One patient with preference for non-operative management also cited the lack of a support system to enable post-operative recovery. Of BMT patients receiving induction chemotherapy, 2 (50%) declined surgical treatment, 1 (25%) expired, and 1 (25%) had poor general condition. Notably, among the 27 patients who met CROSS criteria, we observed an 89% resection rate which compares favorably with the 94% rate noted in the CROSS data and 84% in the post-CROSS data (5,7).
Table 4
| Reason for nonadherence | N, current series | %, Current series | %, Rahmani et al. | %, Depypere et al. |
|---|---|---|---|---|
| Personal choice | 12 | 40.0 | 25.8 | 13.2 |
| Poor general condition | 9 | 30.0 | 12.9 | 22.8 |
| Disease progression | 4 | 13.3 | 32.3 | 43.9 |
| Expired | 2 | 6.7 | 6.5 | 7.9 |
| Unresectable | 2 | 6.7 | – | 12.3 |
| Unresectable intraoperatively | 1 | 3.3 | 14.5 | – |
TMT, trimodality therapy; BMT, bimodality therapy.
Survival
Median follow-up was 69 (95% CI: 58.3–89.9) months. In the overall cohort, median OS was 26.7 (95% CI: 20.3–36.3) months. As shown in Figure 1A, TMT was associated with a significantly higher OS, with median and 3-yr OS at 40.5 months (95% CI: 33.1–83.1) and 62% (95% CI: 46–74%). For patients receiving BMT, median and 3-yr OS was 18.7 months (95% CI: 14.6–22.1) and 18% (95% CI: 7–32%). Similar outcomes were observed when including only patients who did not meet criteria for the CROSS trial, with an observed median OS for TMT of 40.5 (95% CI: 18.4–not reached) months and 19.4 (95% CI: 12.4–22.1) months for BMT, as shown in Figure 1B. As characterized in Figure 1C, for patients declining esophagectomy resulting in BMT, OS was initially similar to TMT patients. However, by 2 years OS was similar to patients with BMT planned from the start of therapy (P=non-significant) while being significantly worse than TMT.
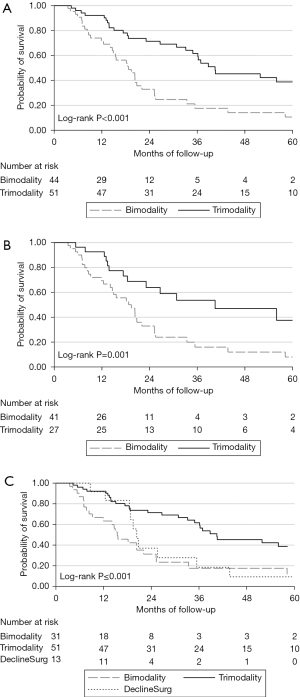
Only treatment modality was associated with improved OS on multivariable Cox regression (TMT vs. BMT: HR 0.34; 95% CI: 0.20–0.57) with 63 death events observed. Univariable and multivariable analysis of OS can be found in the Table S3. Landmark assessments of 6 and 12 months were completed as a sensitivity analysis for immortal time bias introduced by patients who expired during neoadjuvant therapy; however, patterns of OS were similar to analyses of the whole cohort.
RFS was significantly higher for patients receiving TMT with median and 3-yr RFS at 83.1 months (95% CI: 36.9–not reached) and 71% (95% CI: 54–83%) in contrast to 20.3 months (95% CI: 15.4–25.2) and 18% (95% CI: 7–33%) for patients receiving BMT (see Figure 2A). DFS was significantly higher for patients receiving TMT, with median and 3-yr DFS at 38.8 months (95% CI: 30.8–83.1) and 58% (95% CI: 43–71%) in contrast to 18.5 months (95% CI: 12.4–20.4) and 12% (95% CI: 4–24%) among patients receiving BMT (see Figure 2B).

For TMT patients, 30 and 90-day postoperative mortality was low, observed to be 2.0% and 3.9%, respectively. All patients who expired before 90 days (n=2) were ≥69 years old.
Discussion
Survival benefits of TMT
In our dataset, only TMT was associated with improved OS after adjusting for covariates. Even when excluding patients who met published criteria for the CROSS trial, TMT continued to be associated with improved OS. Although these data reflect composite criteria, further characterization of individual criteria may translate into more precise patient selection for TMT.
We believe we have captured a cohort that is more inclusive of patients who are older and more vulnerable compared to available literature, thus making our results potentially more generalizable to the typical range of esophageal cancer patients. For example, in the previously cited “post-CROSS” analysis of consecutive patients regardless of age or performance status, significant differences between the “post-CROSS” and CROSS cohort were of small absolute difference and nearly every patient in the cohort had a Karnofsky Performance Scale ≥90, the mean age was 62, and every patient had an unadjusted CCI ≤2 (7). The post-CROSS cohort reported similar 3-year DFS (54% vs. 58%) and median OS (44.2 vs. 40.5 months) compared to our TMT population, indicating generalizability of expected results (7). Limitations of generalizability for our study are discussed below in ‘limitations’.
Additional analyses of older patients undergoing esophagectomy have similar long-term control benefits of TMT and tolerability of neoadjuvant chemoradiation for selected patients, with some conflicting findings of increased post-operative mortality in elderly patients (defined as ≥65–70 years old) (20-22). In our cohort we observed notably low postoperative mortality despite being a low-volume hospital (~10 esophagectomies/year), with two patients expiring <90 days postoperatively. Both patients were ≥69 years of age, an age group representing 11.8% of TMT patients in our cohort.
Our study period predated standard use of nivolumab for TMT patients based on CheckMate577 trial which found improved DFS with adjuvant nivolumab after TMT; thus, no patients received adjuvant nivolumab in our cohort (23). Integration of neoadjuvant immunotherapy is currently under study in ECOG 2174, a double randomized phase II/III trial evaluating the addition of nivolumab during neoadjuvant chemoradiation and the use of single agent vs. doublet adjuvant immunotherapy (NCT 03604991). Further characterizing those expected to respond to immunotherapy in the neoadjuvant, adjuvant, or definitive treatment setting may further change standard systemic therapy options for esophageal cancer patients (24). Evidence of non-inferiority for peri-operative chemotherapy compared to the CROSS regimen in terms of OS has been observed in the Neoadjuvant trial in Adenocarcinoma of the Esophagus and Esophago-Gastric Junction International Study (NEO-AEGIS) trial, but precise analysis by Siewert class and comparison to survival outcomes with addition of immunotherapy are needed to determine appropriate patients for consideration of omission of radiation and to compare with updated practice following CheckMate577 (25).
Clinical variables associated with BMT
Of the five variables independently associated with BMT on multivariable analysis, four (comorbidities, performance status, N-stage, and chief complaint) are assessable at presentation or with initial work-up. Additionally, held chemotherapy treatments were significantly associated with BMT and were the only treatment variable independently associated with BMT. Notably, these factors were associated with BMT, but were not independently associated with decreased overall survival after adjusting for covariates (including treatment modality).
Prehabilitation therapies administered during neoadjuvant treatment may serve to mitigate the effects of comorbidities and performance status, reduce patient decompensation, and bolster TMT adherence. Previously described interventions include supervised intensive inspiratory muscle training and a “walk and eat” intervention; however, further characterization of interventions and patients who derive benefit is needed, and should be contextualized with adherence to treatment (26,27). For patients presenting with dysphagia, neoadjuvant therapy with either chemoradiation or chemotherapy may provide symptomatic relief and help to bolster nutritional status (28). In patients presenting without dysphagia who develop odynophagia secondary to neoadjuvant therapy, additional nutritional support may be associated with increased rates of resection. Individualized consultation with a dietician following diagnosis may help optimize nutrition status and tolerability of chemoradiation, and should be the standard of care for all patients (29,30).
Interrupted chemotherapy administration may serve as a rough proxy for toxicity status during neoadjuvant chemoradiation. Our study was limited due to insufficient data to assess individual toxicities despite exhaustive review of available records. TMT patients who completed the CROSS LDRT regimen and do not receive a full five cycles of chemotherapy have been observed to have worse overall survival (39 vs. 18 months), although similar outcomes do not appear to be previously studied in BMT (31). Robust prospective data exploring both toxicities and interventions to mitigate their effects on patient’s ability or willingness to undergo esophagectomy is needed. Re-examination of the optimal duration of concurrent chemotherapy may be warranted with the addition of adjuvant nivolumab to the current standard of care.
We did not observe any significant association between tumor histology and modality, possibly due to a large proportion of patients with adenocarcinoma. In patients with squamous cell histology, multiple studies demonstrate noninferiority of BMT compared to TMT (32,33). We included patients with squamous histology in our final analysis, as TMT is the current standard of practice due to the challenges and potential morbidity associated with salvage surgery (2).
Treatment approaches (including radiation dose, chemotherapy regimen, and use of induction chemotherapy) were not significantly associated with treatment modality in our analysis. Thus, these heterogenous approaches were not differentiated. Notably, about 13% of our study cohort received induction chemotherapy as part of a clinical trial. While proceeding to surgery per protocol may have had an impact on treatment course for enrolled patients, we did observe patients disenrolling and declining surgical management after induction chemotherapy.
In a recent systematic review, LDRT in the neoadjuvant setting, most commonly to the CROSS-protocol dose of 41.4 Gy, was found to be associated with significantly improved PFS and OS, improved safety, and lower distant failure rate as well as more favorable side-effect profile compared to higher-dose radiation therapy (HDRT, any dose >48.85 Gy10) (34). Individual studies demonstrate mixed data for differences in pathologic complete response and survival following LDRT compared to HDRT (35,36). While we did not find any association with dose and modality in our series, only 12% (6/51) TMT patients in our series received a neoadjuvant dose of 41.4 Gy, reflecting changing practice over the study period. All 6 patients who received this “CROSS Protocol” of 41.4 Gy proceeded to surgery. This dose a preferred dose per American Radium Society Appropriate Use Criteria guidelines for esophageal adenocarcinoma (1). In patients who are committed to TMT, 41.4 Gy is an appropriate dose with emerging evidence for decreased toxicity with potential to impact adherence, whereas HDRT to 50 or 50.4 Gy may be most appropriate in the definitive setting, including patients undecided about pursuing surgery. Treatment with proton beam therapy (PBT), in comparison to intensity modulated radiation therapy (IMRT), has been observed to be associated with a decrease in the novel metric total toxicity burden (as defined by the authors) with similar PFS and OS (37). However, this study did not utilize LDRT and it is unclear if benefit in toxicity for PBT would be sustained when delivering LDRT in the neoadjuvant setting.
Drivers of TMT nonadherence
In our study, 40% of patients nonadherent to TMT declined surgery. Two prior studies of nonadherence to surgery reported rates of voluntarily declined esophagectomy between 13.2% and 25.8%, with collection periods spanning 2002–2015 and 2007–2016, respectively (11,12). In one study, significant OS benefit was observed for patients declining surgery when there was a high proportion of complete clinical response (11). Patients declining surgery frequently cited quality of life as their chief concern, but follow up was not conducted to determine if patients felt regret over this decision (12).
A prospective discreet-choice experiment including 100 patients 4-6 weeks after completion of neoadjuvant chemoradiation and before surgery found patients would be willing to trade a 5-year OS reduction of 16% if the chance of needing surgery decreased from 100% to 35% through active surveillance (38). We observed similar OS at 24 months between patients who were offered and declined esophagectomy and patients who were nonadherent to TMT for other reasons. Comparing patients declining esophagectomy and patients receiving TMT, we observed an approximately 40% difference in probability of OS between at 3 years, far greater than the acceptable five-year survival difference of 16% in Noordman et al., albeit in a shorter follow-up period.
While the National Comprehensive Cancer Network suggests considering definitive BMT for squamous cell patients if positron emission tomography (PET)/computed tomography (CT) and endoscopy with biopsy show complete response, false negatives may occur and evidence to support active surveillance is still lacking. Radiation Therapy Oncology Group (RTOG) 8501 demonstrated five-year OS of 13% for definitive BMT in patients with adenocarcinoma histology, with only 1 of 23 patients alive with long-term follow up (39). In RTOG 0436, local recurrence rates following definitive BMT approached 50% in both arms, finding no benefit to adding cetuximab and suggesting that many patients recur at a time where development of fibrosis might impair candidacy for salvage esophagectomy (40). Trials have reported rates of resection between 6% and 33% during active surveillance using heterogenous approaches (41). To routinely offer organ-preserving treatment to patients who prioritize OS, strong evidence to support noninferiority of active surveillance is necessary (42). Multiple ongoing trials comparing TMT to active surveillance may provide clarity over the next several years (43-45). Circulating tumor DNA (ctDNA) has demonstrated association with recurrence and survival and may become an additional tool for prognostication and identification of patients for whom nonsurgical management carries lower risk (46,47). Information is needed to characterize the quantity of patients declining esophagectomy beyond single-institution data and comprehensively assess patient decision-making.
Disease progression noted during restaging post-chemoradiation was the most common reason for TMT nonadherence in previous studies (comprising 43.9% and 32.3%) (11,12). We observed far lower rates of TMT nonadherence attributable to disease progression (13.3%). Due to many patients declining surgery in our series, results are skewed away from other factors in favor of personal choice.
Poor general condition was the second highest reason for nonadherence at 30% in our analysis. Our findings are more consistent with the literature for this measure. Prehabilitation, as discussed previously, may reduce decompensation as a driver of nonadherence. Additionally, LDRT has demonstrated superior benefit with improved therapeutic ratio and should be considered to support patients in completing TMT (34).
Limitations
Limitations include those inherent to retrospective analyses. Retrospective analyses present challenges when controlling for differences between cohorts to mitigate effects of selection bias. Through multivariable analysis, we controlled for differences in common prognostic factors between patients receiving TMT and BMT when analyzing for association with survival and other outcomes. Additionally, our exclusion criteria were designed to create a cohort in which patient presentation was both inclusive of patients who were older and with increased frailty, but minimized uncontrollable variation in disease through exclusion of patients receiving treatment without curative intent. However, some patients may have experienced inferior outcomes due to factors for which we did not collect data or had incomplete data, or had complexities difficult to characterize and study in a single-institution retrospective cohort, which may impact the results and associated conclusions of our multivariable analyses and these should be interpreted accordingly.
Our analysis was limited to the content and quality of available data, which commonly impacts analyses of RWD (48). The power of our study was determined by available data, and may have impacted our ability to detect the association of factors additional to treatment modality with overall survival. For patients who received parts of their care outside of our cancer center, we had limited access to records; however, through the inclusion of patients who received part of their care at a high-volume cancer center, we hope to provide an analysis that is both representative of the patient population at a center with smaller volume and inclusive of patients without means to access a high-volume center. Overall, limited data were available to assess individual treatment toxicities. Few patients received PET scans following neoadjuvant therapies in our cohort; thus, we were unable to analyze PET response for association with BMT or OS.
We included patients who met inclusion criteria but planned for definitive BMT at the start of therapy in our analyses for association of clinical factors with modality and our analyses of survival, but excluded these patients from analyses of adherence. While we were able to record clearly documented plans for definitive BMT, many patients had contingent plans and patients were offered initial consultation with a surgeon at varying timepoints relative to diagnosis and therapy during our study period. For these reasons, analyses of survival based on intent to treat with definitive BMT as compared to the remainder of our cohort is of limited utility and was not included in our findings.
Of patients receiving BMT, two expired during the anticipated nCRT course and one expired during the interval between nCRT and surgery, which may introduce immortal time bias in analyses of modality for association with survival; however, landmark assessments at 6 and 12 months suggest against immortal time bias in our results.
Conclusions
Trimodality therapy was associated with an improved OS, RFS, and DFS for patients with resectable esophageal cancer in our cohort. Further characterizing the patients who derive the greatest benefit from esophagectomy is necessary. Interventions during neoadjuvant therapy may be an important area for future research efforts to identify ways to best physiologically prepare patients to receive surgery. Furthermore, patient preference for organ-preserving therapies appears to have a significant impact on resection rate in our cohort; further characterization of patient decision-making may be helpful to best counsel patients when weighing potential OS benefits versus treatment morbidity. Patients should be given the option to discuss details regarding their expected prognosis with TMT vs. BMT, so that they better understand the long-term expectations of both treatment modalities. Therefore, early consultation with a surgeon should be facilitated for all patients with potentially resectable disease. For those patients who wish to pursue TMT, the CROSS protocol involving 41.4 Gy/23 fractions (rather than 50.4 Gy/28 fractions) should be considered to minimize treatment toxicity and support TMT adherence.
Acknowledgments
Preliminary findings were presented in poster format at the New England Clinical Oncologic Society (NNECOS) Annual Meeting as well as the American Society for Radiation Oncology Annual Meeting.
Funding: This work was supported by NNECOS and the University of Vermont Larner College of Medicine (UVM LCOM) Summer Research Fellowship.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-633/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-633/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-633/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-633/coif). All authors report that this study received financial support from NNECOS and the UVM LCOM Summer Research Fellowship. Any opinions, findings, and conclusions expressed in this material are those of the author(s) and do not necessarily reflect those of the NNECOS. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional review board of the University of Vermont (IRB00000485) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Anker CJ, Dragovic J, Herman JM, et al. Executive Summary of the American Radium Society Appropriate Use Criteria for Operable Esophageal and Gastroesophageal Junction Adenocarcinoma: Systematic Review and Guidelines. Int J Radiat Oncol Biol Phys 2021;109:186-200. [Crossref] [PubMed]
- Ajani JA, D'Amico TA, Bentrem DJ, et al. Esophageal and Esophagogastric Junction Cancers, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2019;17:855-83. [Crossref] [PubMed]
- Noordman BJ, Verdam MGE, Lagarde SM, et al. Effect of Neoadjuvant Chemoradiotherapy on Health-Related Quality of Life in Esophageal or Junctional Cancer: Results From the Randomized CROSS Trial. J Clin Oncol 2018;36:268-75. [Crossref] [PubMed]
- Eyck BM, van Lanschot JJB, Hulshof MCCM, et al. Ten-Year Outcome of Neoadjuvant Chemoradiotherapy Plus Surgery for Esophageal Cancer: The Randomized Controlled CROSS Trial. J Clin Oncol 2021;39:1995-2004. [Crossref] [PubMed]
- Shapiro J, van Lanschot JJB, Hulshof MCCM, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090-8. [Crossref] [PubMed]
- Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol 2008;26:1086-92. [Crossref] [PubMed]
- Toxopeus E, van der Schaaf M, van Lanschot J, et al. Outcome of Patients Treated Within and Outside a Randomized Clinical Trial on Neoadjuvant Chemoradiotherapy Plus Surgery for Esophageal Cancer: Extrapolation of a Randomized Clinical Trial (CROSS). Ann Surg Oncol 2018;25:2441-8. [Crossref] [PubMed]
- Hong JC. Strategies to Turn Real-world Data Into Real-world Knowledge. JAMA Netw Open 2021;4:e2128045. [Crossref] [PubMed]
- Penberthy LT, Rivera DR, Lund JL, et al. An overview of real-world data sources for oncology and considerations for research. CA Cancer J Clin 2022;72:287-300. [Crossref] [PubMed]
- Verma V, Haque W, Zheng D, et al. Patterns of Care and Outcomes of Elderly Esophageal Cancer Patients Not Meeting Age-based Criteria of the CROSS Trial. Am J Clin Oncol 2019;42:67-74. [Crossref] [PubMed]
- Depypere L, Thomas M, Moons J, et al. Analysis of patients scheduled for neoadjuvant therapy followed by surgery for esophageal cancer, who never made it to esophagectomy. World J Surg Oncol 2019;17:89. [Crossref] [PubMed]
- Rahmani R, Koffler D, Haisley KR, et al. Stop hedging your bets: reasons for non-adherence to a tri-modality regimen in the treatment of esophageal cancer in a multidisciplinary setting. J Gastrointest Oncol 2019;10:387-90. [Crossref] [PubMed]
- Siewert JR, Stein HJ. Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg 1998;85:1457-9. [Crossref] [PubMed]
- Rice TW, Patil DT, Blackstone EH. 8th edition AJCC/UICC staging of cancers of the esophagus and esophagogastric junction: application to clinical practice. Ann Cardiothorac Surg 2017;6:119-30.
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials 1996;17:343-6. [Crossref] [PubMed]
- Clark TG, Bradburn MJ, Love SB, et al. Survival analysis part I: basic concepts and first analyses. Br J Cancer 2003;89:232-8. [Crossref] [PubMed]
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. [Crossref] [PubMed]
- [Dataset] Luke Higgins; 2019-2022; Institutional Resectable Esophageal Cancer Dataset; in institutional repository.
- Camerlo A, D'Journo XB, Ouattara M, et al. Adenocarcinoma of the esophagus and esophagogastric junction in patients older than 70 years: results of neoadjuvant radiochemotherapy followed by transthoracic esophagectomy. J Visc Surg 2012;149:e203-10. [Crossref] [PubMed]
- Horne ZD, Wegner RE, Colonias A, et al. Drivers of 30- and 90-day Postoperative Death After Neoadjuvant Chemoradiation for Esophageal Cancer. Ann Thorac Surg 2020;109:921-6. [Crossref] [PubMed]
- Lester SC, Lin SH, Chuong M, et al. A Multi-institutional Analysis of Trimodality Therapy for Esophageal Cancer in Elderly Patients. Int J Radiat Oncol Biol Phys 2017;98:820-8. [Crossref] [PubMed]
- Kelly RJ, Ajani JA, Kuzdzal J, et al. Adjuvant Nivolumab in Resected Esophageal or Gastroesophageal Junction Cancer. N Engl J Med 2021;384:1191-203. [Crossref] [PubMed]
- Hou S, Pan Z, Hao X, et al. Recent Progress in the Neoadjuvant Treatment Strategy for Locally Advanced Esophageal Cancer. Cancers (Basel) 2021;13:5162. [Crossref] [PubMed]
- Reynolds JV, Preston SR, O’Neill B, et al. Neo-AEGIS (Neoadjuvant trial in Adenocarcinoma of the Esophagus and Esophago-Gastric Junction International Study): Preliminary results of phase III RCT of CROSS versus perioperative chemotherapy (Modified MAGIC or FLOT protocol). (NCT01726452). J Clin Oncol 2021;39:4004. [Crossref]
- Bolger JC, Loughney L, Tully R, et al. Perioperative prehabilitation and rehabilitation in esophagogastric malignancies: a systematic review. Dis Esophagus 2019;32:doz058. [Crossref] [PubMed]
- Xu YJ, Cheng JC, Lee JM, et al. A Walk-and-Eat Intervention Improves Outcomes for Patients With Esophageal Cancer Undergoing Neoadjuvant Chemoradiotherapy. Oncologist 2015;20:1216-22. [Crossref] [PubMed]
- Sunde B, Johnsen G, Jacobsen AB, et al. Effects of neoadjuvant chemoradiotherapy vs chemotherapy alone on the relief of dysphagia in esophageal cancer patients: secondary endpoint analysis in a randomized trial. Dis Esophagus 2019; [Crossref] [PubMed]
- Isenring EA, Bauer JD, Capra S. Nutrition support using the American Dietetic Association medical nutrition therapy protocol for radiation oncology patients improves dietary intake compared with standard practice. J Am Diet Assoc 2007;107:404-12. [Crossref] [PubMed]
- Movahed S, Seilanian Toussi M, et al. Effects of medical nutrition therapy compared with general nutritional advice on nutritional status and nutrition-related complications in esophageal cancer patients receiving concurrent chemoradiation: A randomized controlled trial. Mediterranean Journal of Nutrition and Metabolism 2020;13:265-76. [Crossref]
- Cloos-V Balen M. Neoadjuvant chemoradiotherapy followed by resection for esophageal cancer: clinical outcomes with the 'CROSS-regimen' in daily practice. Dis Esophagus 2022;35:doab068. [Crossref] [PubMed]
- Bedenne L, Michel P, Bouché O, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol 2007;25:1160-8. [Crossref] [PubMed]
- Stahl M, Stuschke M, Lehmann N, et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol 2005;23:2310-7. [Crossref] [PubMed]
- Li Y, Liu H, Sun C, et al. Comparison of Clinical Efficacy of Neoadjuvant Chemoradiation Therapy Between Lower and Higher Radiation Doses for Carcinoma of the Esophagus and Gastroesophageal Junction: A Systematic Review. Int J Radiat Oncol Biol Phys 2021;111:405-16. [Crossref] [PubMed]
- Nehlsen AD, Lehrer EJ, Resende-Salgado L, et al. Comparison of Pathologic Complete Response Rates and Oncologic Outcomes in Patients With Surgically Resectable Esophageal Cancer Treated With Neoadjuvant Chemoradiation to 50.4 Gy vs 41.4 Gy. Cureus 2021;13:e19233. [Crossref] [PubMed]
- Duque-Santana V, López-Campos F, Martin M, et al. Dose-escalated neoadjuvant chemoradiotherapy for locally advanced oesophageal or oesophagogastric junctional adenocarcinoma. Rep Pract Oncol Radiother 2022;27:500-8. [Crossref] [PubMed]
- Lin SH, Hobbs BP, Verma V, et al. Randomized Phase IIB Trial of Proton Beam Therapy Versus Intensity-Modulated Radiation Therapy for Locally Advanced Esophageal Cancer. J Clin Oncol 2020;38:1569-79. [Crossref] [PubMed]
- Noordman BJ, de Bekker-Grob EW, Coene PPLO, et al. Patients' preferences for treatment after neoadjuvant chemoradiotherapy for oesophageal cancer. Br J Surg 2018;105:1630-8. [Crossref] [PubMed]
- Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA 1999;281:1623-7. [Crossref] [PubMed]
- Suntharalingam M, Winter K, Ilson D, et al. Effect of the Addition of Cetuximab to Paclitaxel, Cisplatin, and Radiation Therapy for Patients With Esophageal Cancer: The NRG Oncology RTOG 0436 Phase 3 Randomized Clinical Trial. JAMA Oncol 2017;3:1520-8. [Crossref] [PubMed]
- Hipp J, Nagavci B, Schmoor C, et al. Post-Neoadjuvant Surveillance and Surgery as Needed Compared with Post-Neoadjuvant Surgery on Principle in Multimodal Treatment for Esophageal Cancer: A Scoping Review. Cancers (Basel) 2021;13:429. [Crossref] [PubMed]
- Noordman BJ, Wijnhoven BPL, Lagarde SM, et al. Active surveillance in clinically complete responders after neoadjuvant chemoradiotherapy for esophageal or junctional cancer. Dis Esophagus 2017;30:1-8. [Crossref] [PubMed]
- Jia R, Yin W, Li S, et al. Chemoradiation versus oesophagectomy for locally advanced oesophageal cancer in Chinese patients: study protocol for a randomised controlled trial. Trials 2019;20:206. [Crossref] [PubMed]
- Noordman BJ, Wijnhoven BPL, Lagarde SM, et al. Neoadjuvant chemoradiotherapy plus surgery versus active surveillance for oesophageal cancer: a stepped-wedge cluster randomised trial. BMC Cancer 2018;18:142. [Crossref] [PubMed]
- Comparison of Systematic Surgery Versus Surveillance and Rescue Surgery in Operable Oesophageal Cancer With a Complete Clinical Response to Radiochemotherapy (Esostrate). Available online: https://clinicaltrials.gov/ct2/show/NCT02551458
- Hofste LSM, Geerlings MJ, von Rhein D, et al. Circulating Tumor DNA-Based Disease Monitoring of Patients with Locally Advanced Esophageal Cancer. Cancers (Basel) 2022;14:4417. [Crossref] [PubMed]
- Yu E, Allan AL, Sanatani M, et al. Circulating tumor cells detected in follow-up predict survival outcomes in tri-modality management of advanced non-metastatic esophageal cancer: a secondary analysis of the QUINTETT randomized trial. BMC Cancer 2022;22:746. [Crossref] [PubMed]
- Yang DX, Miccio JA, Jairam V, et al. The Impact of Missing/Incomplete Data in Real-World Data Studies. Int J Radiat Oncol Biol Phys 2020;108:e394. [Crossref]


