
Diagnostic value of liver contrast-enhanced ultrasound in early hepatocellular carcinoma: a systematic review and meta-analysis
Highlight box
Key findings
• This meta-analysis showed that contrast-enhanced ultrasound (CEUS) of the liver has certain advantages in the early diagnosis of hepatocellular carcinoma and has high sensitivity and specificity.
What is known and what is new?
• CEUS can be performed at the same time as unenhanced ultrasound; thus, it is feasible as a rapid examination for the early diagnosis of hepatocellular carcinoma. The value of CEUS in the diagnosis of hepatocellular carcinoma is still controversial.
• We conducted a meta-analysis to explore the application value of CEUS in the early diagnosis of hepatocellular carcinoma and provide medical evidence for its use in the early diagnosis of hepatocellular carcinoma.
What is the implication, and what should change now?
• CEUS of the liver has certain advantages in the early diagnosis of hepatocellular carcinoma and has clinical application value.
Introduction
Hepatocellular carcinoma is the sixth most common cancer worldwide and a common cause of cancer-related death (1,2). It has been reported that there are about 6.35 million new cases of malignant tumors in the world every year, among which, there are about 260,000 cases of primary hepatic carcinoma, which account for 4.1% of all tumors (3,4). The incidence of hepatocellular carcinoma is relatively high, especially in some developing countries, for which the incidence is 2–3 times that of western countries, and the incidence of hepatocellular carcinoma continues to increase (5). China has a high-incidence of hepatocellular carcinoma.
Because the symptoms of hepatocellular carcinoma are not obvious in the early stage of the disease, patients often have advanced hepatocellular carcinoma at the time of diagnosis. The disease is characterized by a relatively short course of disease and poor prognosis, and has a serious negative impact on public health (6,7). Early diagnosis is one of the important measures to prevent hepatocellular carcinoma and improve the survival rate of hepatocellular carcinoma patients. Thus, a simple, convenient, and rapid diagnosis method needs to be developed to enable the early prevention and treatment of hepatocellular carcinoma.
The occurrence of hepatocellular carcinoma is generally accompanied by changes in the hemodynamics of the lesion. During the transition from cirrhotic nodules to dysplastic nodules and small hepatocellular carcinomas, the blood supply of the nutrient arteries in the nodules gradually increases, while the blood supply of the original portal vein gradually decreases (5). Commonly used imaging examinations for hepatocellular carcinoma include ultrasound, computed tomography, and magnetic resonance imaging. Ultrasound is a safe, common and cost-effective screening test for hepatocellular carcinoma. However, due to differences in the manifestations of hepatocellular carcinoma, the accuracy of ultrasound diagnosis is only 53–77%, and the specificity of ultrasound is not high; thus, the use of ultrasound is limited (8).
Contrast-enhanced ultrasound (CEUS) refers to the addition of contrast-agent microbubbles to conventional ultrasound. The characteristics of the contrast agent can be used to better display the difference between the blood flow in the lesion and the adjacent tissue. Contrast-enhanced ultrasound combined with the characteristics of blood flow changes in hepatocellular carcinoma can better improve the accuracy of hepatocellular carcinoma diagnosis. There is a relatively large difference in blood perfusion between benign and malignant hepatocellular carcinoma, so CEUS can also provide a diagnostic basis for differentiating between benign and malignant hepatocellular carcinoma (9,10). CEUS can also be performed simultaneously with unenhanced ultrasound. Thus, it is feasible as a method for the rapid and early diagnosis of hepatocellular carcinoma.
However, the value of CEUS in the diagnosis of hepatocellular carcinoma is controversial. For example, CEUS has been reported to provide false positive diagnoses of hepatocellular carcinoma in patients with cholangiocarcinoma (11). The detection rate of liver CEUS in detecting early hepatocellular carcinoma has been questioned, and it is still necessary to combine computed tomography (CT), magnetic resonance imaging (MRI), and some biomarkers to improve the detection rate (12). However, some researchers have found that MRI has higher resolution and contrast enhancement function compared to ultrasound and CT, and it is recommended to be the preferred imaging method for hepatocellular carcinoma diagnosis (13). Therefore, in order to verify the application value of CEUS in early hepatocellular carcinoma diagnosis. Thus, in this study, we sought to explore the application value of CEUS in the early diagnosis of hepatocellular carcinoma by using a meta-analysis and provide evidence-based medical evidence for the early diagnosis of hepatocellular carcinoma. We present the following article in accordance with the PRISMA reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-211/rc).
Methods
Literature search
Articles about the use of CEUS for the early diagnosis of hepatocellular carcinoma were retrieved from the PubMed, Cochrane Library, Embase, Ovid Technologies (OVID), China National Knowledge Infrastructure (CNKI), Chongqing VIP Information (VIP), and Wanfang databases. The search was carried out by combining subject terms with free words. The Chinese and English keywords included contrast-enhanced ultrasound, early hepatocellular carcinoma, hepatocellular carcinoma, diagnosis, application, and value. The retrieval time was from the establishment of the databases to January 2023.
Article inclusion and exclusion criteria
Inclusion criteria
To be eligible for inclusion in this meta-analysis, the articles had to meet the following inclusion criteria: (I) include patients aged ≥18 years with liver nodules undergoing hepatocellular carcinoma screenings as the research objects; (II) use pathological diagnosis as the gold standard of diagnosis; (III) be an original Chinese- or English-language article; and (IV) indirectly or directly include primitive numbers, such as true positive numbers, false positive numbers, false negative numbers, and true negative numbers.
Exclusion criteria
Articles were excluded from the meta-analysis if they met any of the following exclusion criteria: (I) the original data were missing, could not be extracted from the article or were not available; (II) the article concerned a review, master’s or doctoral dissertation, conference, or other type of document; and/or (III) the intervention measures did not include CEUS or CEUS combined with other measures.
Article screening and data extraction
Two researchers independently screened the articles according to the inclusion and exclusion criteria. The data extracted included the general data and outcome data. The general data included the authors and publication year, country, number of cases, number of lesions, age of patients, and size of nodules. The outcome data included the number of true positives, false positives, false negatives, and true negatives of diagnosis.
Literature quality evaluation
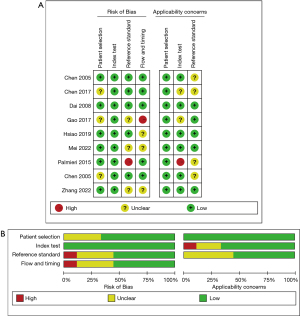
The article quality evaluation was carried out using the Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) (14). The included articles were evaluated in Review Manager 5.3 (Cochrane collaboration network), which already included the evaluation tool. Each item was rated “Yes”, “No”, or “Unclear”, and a document quality evaluation map was drawn. If all entries match, it is considered a low bias risk, some matches represent an unclear bias risk, and all non matches represent a high bias risk.
Statistical analysis
The statistical analysis was performed using Stata 17.0 (Computer Resource Center, USA). A bivariate mixed-effects model was fitted. The sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic odds ratio (DOR), and the corresponding 95% confidence interval (CI) were calculated. A summary receiver operating characteristic curve was drawn, and the area under the curve (AUC) with the 95% CI was calculated (15). The publication bias of the included studies was assessed by Deek’s funnel plot asymmetry test. If the P value was <0.05, there was an obvious publication bias in the article. The Q test and I2 were used to determine heterogeneity. If the P value was <0.1 and the I2 value was >50%, the heterogeneity was significant, and a meta-regression was used to explore the source of heterogeneity. A two-sided P value <0.05 was considered statistically significant.
Results
Literature search results
A total of 945 articles were retrieved from the databases, including 763 English-language articles and 182 Chinese-language articles. After removing the duplicate documents, 724 articles remained. After reading the titles and abstracts, 73 articles remained. After downloading the full text, 71 articles remained. Among these articles, 16 without a negative control group were excluded, 8 that did not match the research type were excluded, 29 with missing important data were excluded, and 9 that examined co-infections were excluded. Ultimately, 9 articles (5,6,16-22) were included in the meta-analysis. The screening process is shown in Figure 1.
Basic information of the included articles and quality evaluation of the literature
The general information of the included articles is detailed in Table 1. The quality evaluation results of QUADAS-2 are shown in Figure 2. Of the 9 articles, 3 did not clarify whether the case inclusion method was continuous or random (16,19,22). The experimental results of all the articles were interpretated based on the premise that the results of the gold standard method were known (5,6,16-22). However, 3 articles did not state whether the blind method was used in the judgment of results (16,19,21). Additionally, 3 articles had inappropriate exclusion of experimental group or control group cases (16,17,21). The time interval between the interpretation of the results of the experimental group and the gold standard method in the 4 articles was long (5,6,19,20). In summary, only 1 out of 9 articles has a low risk of bias, while the remaining 8 articles have an unclear risk of bias.
Table 1
| First author and year published | Country | Number of patients | Number of lesions | Age (years) | Sex ratio (male/female) | Nodule size (cm) |
|---|---|---|---|---|---|---|
| Chen 2005, (17) | China | 75 | 180 | 59.2±6.8 | 39/36 | 0.7–3.1 |
| Chen 2017, (16) | China | 392 | – | >55 | – | <2 |
| Dai 2008, (18) | China | 35 | – | – | – | <2 |
| Gao 2017, (19) | China | 215 | 236 | 57.6±10.6 | 136/79 | <3 |
| Hsiao 2019, (20) | China | 66 | – | 63.3±9.3 | 45/21 | 1.6±0.7 |
| Mei 2022, (5) | China | 395 | 632 | 54.7±12.8 | 230/165 | ≤2 |
| Palmieri 2015, (21) | Italy | 124 | 148 | 46–97 | 96/28 | ≤2 |
| Chen 2005, (22) | China | 48 | 146 | – | – | <3 |
| Zhang 2022, (6) | China | 84 | – | ≥18 | 54/30 | <3 |
The age and nodule size in the table are represented by mean ± SD or range. SD, standard difference.
CEUS in the early diagnosis of hepatocellular carcinoma
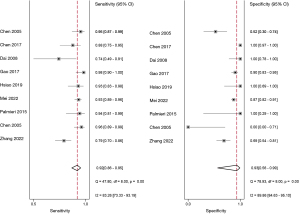
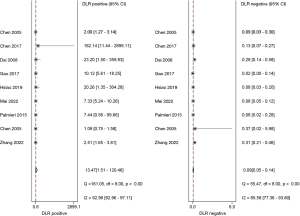
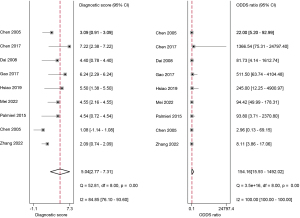
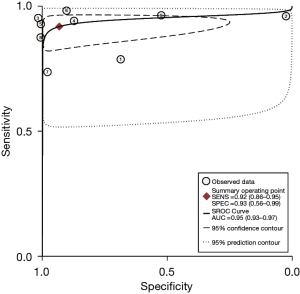
The studies ultimately included in the meta-analysis were analyzed using a bivariate mixed-effects model. The CEUS had a pooled sensitivity of 0.92 (95% CI: 0.86–0.95), a pooled specificity of 0.93 (95% CI: 0.56–0.99), a pooled PLR of 13.47 (95% CI: 1.51–120.46), a pooled NLR of 0.09 (95% CI: 0.05–0.14), a combined DOR of 154.16 (95% CI: 15.93–1,492.02), a diagnostic score of 5.04 (95% CI: 0.277–7.31), and a pooled AUC of 0.95 (95% CI: 0.93–0.97). The heterogeneity test results showed that I2>50% and P<0.1 (Figures 3-6).
Threshold effect
A Spearman correlation analysis was carried out using the logSENS and Log(1-SENS). The r correlation coefficient result was 0.13 (P>0.05). There was no threshold effect in this study. The source of heterogeneity was not the threshold effect.
Analysis of sources of heterogeneity
A meta-regression was used to find the source of heterogeneity of the included studies. The results showed that the country of publication (P=0.14) and the size of the lesions and nodules (P=0.46) were not sources of heterogeneity. Therefore, possible reasons can only be inferred from the literature. After re-analysis of the literature, we found that the heterogeneity of the analysis results may be caused by the differences in the detection instruments used by the medical units and the technical proficiency of the operators.
Publication bias
Deek’s funnel plot asymmetry test was used to test the publication bias of the included articles. A P value of 0.74 was found, which indicated that there was no potential publication bias in the included articles (Figure 7).
Discussion
Hepatocellular carcinoma is a malignant tumor with high clinical mortality. According to epidemiological data, China has a high incidence of hepatocellular carcinoma. Thus, the current situation in relation to the prevention and treatment of hepatocellular carcinoma in China is still very serious. Hepatocellular carcinoma has gradually become a public health problem that threatens the health and quality of life of residents (6,23,24). A large number of studies have shown that the disease progression of hepatocellular carcinoma is faster than that of other malignant tumors (25-27). This is related to the dual blood supply to the liver by the hepatic artery and portal vein (25,27).
Surgery is the main means of radical cure for hepatocellular carcinoma. However, the onset of hepatocellular carcinoma is hidden, and the disease develops rapidly in the later stages. Some patients are already in the advanced stages of hepatocellular carcinoma when they develop symptoms. Surgical treatment is difficult for such patients, who may even miss the opportunity to undergo surgery (28,29). Thus, early diagnosis is of great significance if the success rates of surgery and prognosis are to be improved. Recent studies have reported that the early diagnosis of hepatocellular carcinoma and radical surgery for hepatocellular carcinoma significantly improves the 5-year survival rate of patients after surgery (7,30). However, the experience and abilities of different radiologists can lead to different outcomes even for the same lesion. Although standardized testing can minimize subjectivity, the impact of subjectivity is still inevitable due to the differences and complexity between individuals, and meta-analysis is a very applicable method for resolving controversial issues.
This study examined 9 articles on the use of CEUS in the early diagnosis of hepatocellular carcinoma. The meta-analysis results showed that the combined sensitivity and specificity of CEUS were 0.92 (95% CI: 0.86–0.95) and 0.93 (95% CI: 0.56–0.99), respectively. Thus, CEUS had high sensitivity and specificity in diagnosing early hepatocellular carcinoma. CEUS had an AUC of 0.95 (95% CI: 0.93–0.97), and a diagnostic score of 5.04 (95% CI: 0.277–7.31). This result indicates that CEUS is a highly specific diagnostic system and a better tool to avoid misdiagnosing non-HCC observations as HCC. However, it is not possible to reach 1, which means that there may still be missed diagnoses of HCC (31). The heterogeneity test results showed that there was heterogeneity in the included articles (I2>50% and P<0.1), but the correlation coefficient of the threshold-effect analysis was 0.13 (P>0.05). Thus, the threshold effect was not the source of heterogeneity. A meta-regression was then used to analyze the sources of heterogeneity. However, the results showed that neither the country of publication (P=0.14) nor the size of lesions and nodules (P=0.46) were sources of heterogeneity. The above results also indicate that there are still many issues that have not been clarified in the field of early hepatocellular carcinoma diagnosis using liver ultrasound. Therefore, more research and analysis are needed for further exploration.
This meta-analysis had some limitations. First, many factors affect the accuracy of CEUS, such as differences in operators’ technical proficiency, and differences in the use of instruments, which may have affected the heterogeneity of the included articles. Second, some of the 9 articles included in this analysis were missing the baseline data of patients. Thus, there were relatively few factors that could be analyzed in the meta-regression analysis, which had a certain effect on the heterogeneity analysis. Third, some of the results of the QUADAS-2 quality assessment were “unclear,” which brings a risk of bias.
In summary, CEUS has certain advantages in the early diagnosis of hepatocellular carcinoma, and has high sensitivity and specificity. CEUS has clinical application value.
Conclusions
Hepatic CEUS has certain advantages in the diagnosis of early hepatocellular carcinoma due to its high sensitivity and specificity. However, considering some limitations in the analysis in this article, further research and analysis are needed to explore this conclusion.
Acknowledgments
Funding: This study was supported by the Hainan Natural Science Foundation Project Support (No. 819MS140).
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-211/rc
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-211/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-211/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of this work, including ensuring that any questions related to the accuracy or integrity of any part of the work have been appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Ayuso C, Rimola J, Vilana R, et al. Diagnosis and staging of hepatocellular carcinoma (HCC): current guidelines. Eur J Radiol 2018;101:72-81. [Crossref] [PubMed]
- Schwarze V, Marschner C, Völckers W, et al. The diagnostic performance of contrast-enhanced ultrasound (CEUS) for evaluating hepatocellular carcinoma (HCC) juxtaposed to MRI findings; a retrospective single-center analysis of 292 patients. Clin Hemorheol Microcirc 2020;76:155-60. [Crossref] [PubMed]
- Ellebaek SB, Fristrup CW, Pless T, et al. The value of contrast-enhanced laparoscopic ultrasound during robotic-assisted surgery for primary colorectal cancer. J Clin Ultrasound 2018;46:178-82. [Crossref] [PubMed]
- Mei Q, Yu M, Chen Q. Clinical value of contrast-enhanced ultrasound in early diagnosis of small hepatocellular carcinoma (≤ 2 cm). World J Clin Cases 2022;10:8525-34. [Crossref] [PubMed]
- Zhang L, Gu J, Li Y, et al. Clinical Value Study on Contrast-Enhanced Ultrasound Combined with Enhanced CT in Early Diagnosis of Primary Hepatic Carcinoma. Contrast Media Mol Imaging 2022;2022:7130533. [Crossref] [PubMed]
- Della Corte C, Triolo M, Iavarone M, et al. Early diagnosis of liver cancer: an appraisal of international recommendations and future perspectives. Liver Int 2016;36:166-76. [Crossref] [PubMed]
- Singal A, Volk ML, Waljee A, et al. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther 2009;30:37-47. [Crossref] [PubMed]
- Beyer LP, Pregler B, Wiesinger I, et al. Continuous dynamic registration of microvascularization of liver tumors with contrast-enhanced ultrasound. Radiol Res Pract 2014;2014:347416. [Crossref] [PubMed]
- Zhou LQ, Wang JY, Yu SY, et al. Artificial intelligence in medical imaging of the liver. World J Gastroenterol 2019;25:672-82. [Crossref] [PubMed]
- Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020-2. [Crossref] [PubMed]
- Kong Y, Jing Y, Sun H, et al. The Diagnostic Value of Contrast-Enhanced Ultrasound and Enhanced CT Combined with Tumor Markers AFP and CA199 in Liver Cancer. J Healthc Eng 2022;2022:5074571. [Crossref] [PubMed]
- Shen X, Wu J, Su J, et al. Revisiting artificial intelligence diagnosis of hepatocellular carcinoma with DIKWH framework. Front Genet 2023;14:1004481. [Crossref] [PubMed]
- Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529-36. [Crossref] [PubMed]
- Rosman AS, Korsten MA. Application of summary receiver operating characteristics (sROC) analysis to diagnostic clinical testing. Adv Med Sci 2007;52:76-82. [PubMed]
- Chen M, Wu W. Application value of contrast-enhanced ultrasound in the early diagnosis of cirrhosis combined with small hepatocellular carcinoma. Chinese Journal of Practical Medicine 2017;44:103-4.
- Chen MH, Dai Y, Yan K, et al. Early diagnosis of small hepatocellular carcinoma by new contrast-enhanced ultrasound technique. Beijing Da Xue Xue Bao Yi Xue Ban 2005;37:458-62. [PubMed]
- Dai L, Feng X, Chen Y, et al. Imaging diagnosis of small hepatocellular carcinoma using ultrasound, contrast-enhanced ultrasound and multislice spiral CT. Nan Fang Yi Ke Da Xue Xue Bao 2008;28:1469-71. [PubMed]
- Gao Y, Li M, Chen M, et al. Clinical value of contrast-enhanced ultrasound in the diagnosis of early liver cancer. Chinese Journal of the Frontiers of Medical Science 2017;9:87-90.
- Hsiao CY, Chen PD, Huang KW. A Prospective Assessment of the Diagnostic Value of Contrast-Enhanced Ultrasound, Dynamic Computed Tomography and Magnetic Resonance Imaging for Patients with Small Liver Tumors. J Clin Med 2019;8:1353. [Crossref] [PubMed]
- Palmieri VO, Santovito D, Marano G, et al. Contrast-enhanced ultrasound in the diagnosis of hepatocellular carcinoma. Radiol Med 2015;120:627-33. [Crossref] [PubMed]
- Chen MH, Dai Y, Yan K, et al. Early diagnosis of small hepatocellular carcinoma in patients with cirrhosis using contrast-enhanced ultrasound. Chinese Journal of Ultrasonography 2005;35-9.
- Ye T, Shao SH, Ji K, et al. Evaluation of short-term effects of drug-loaded microspheres and traditional transcatheter arterial chemoembolization in the treatment of advanced liver cancer. World J Gastrointest Oncol 2022;14:2367-79. [Crossref] [PubMed]
- Zhao M, Wang Y, Zhang Y, et al. The upregulation of stromal antigen 3 expression suppresses the phenotypic hallmarks of hepatocellular carcinoma through the Smad3-CDK4/CDK6-cyclin D1 and CXCR4/RhoA pathways. BMC Gastroenterol 2022;22:378. [Crossref] [PubMed]
- Riviere DM, van Geenen EJM, van der Kolk BM, et al. Improving preoperative detection of synchronous liver metastases in pancreatic cancer with combined contrast-enhanced and diffusion-weighted MRI. Abdom Radiol (NY) 2019;44:1756-65. [Crossref] [PubMed]
- Liu WQ, Li WL, Ma SM, et al. Discovery of core gene families associated with liver metastasis in colorectal cancer and regulatory roles in tumor cell immune infiltration. Transl Oncol 2021;14:101011. [Crossref] [PubMed]
- Garcia-Carbonero R. SEOM clinical guidelines for the diagnosis and treatment of gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs) 2014. Clin Transl Oncol 2014;16:1025-34. [Crossref] [PubMed]
- Wang B, Min W, Lin S, et al. Saikosaponin-d increases radiation-induced apoptosis of hepatoma cells by promoting autophagy via inhibiting mTOR phosphorylation. Int J Med Sci 2021;18:1465-73. [Crossref] [PubMed]
- Huang J, Duan Q, Fan P, et al. Clinical evaluation of targeted arterial infusion of verapamil in the interventional chemotherapy of primary hepatocellular carcinoma. Cell Biochem Biophys 2011;59:127-32. [Crossref] [PubMed]
- Kudo M. Diagnostic imaging of hepatocellular carcinoma: recent progress. Oncology 2011;81:73-85. [Crossref] [PubMed]
- Peng J, Zhang T, Wang H, et al. The Value of Contrast-Enhanced Ultrasound Liver Imaging Reporting and Data System in the Diagnosis of Hepatocellular Carcinoma: A Meta-Analysis. J Ultrasound Med 2022;41:1537-47. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)




