
Advances in pre-treatment evaluation of pancreatic ductal adenocarcinoma: a narrative review
Introduction
Background
Pancreatic ductal adenocarcinoma (PDAC) is one of the most lethal malignancies worldwide, because of difficulties in diagnosis, early tumour metastasis and tumour refractoriness to existing therapies. Incidence continues to increase in both men and women. For pancreatic cancer, 62,210 new diagnoses and 49,830 deaths are expected in 2022 in the United States. It accounts for 8% of all cancer-related deaths, ranking fourth for both sexes (1). It has been projected that pancreatic cancer will be the second leading cause of cancer-related death in the United States in 2030, second only to lung cancer, surpassing breast cancer as the third cause of cancer death in the European Union (2).
The most important factor affecting survival is the staging at diagnosis. Only 20% of patients are resectable at diagnosis and their 5-year overall survival (OS) is estimated at 27% (3). To date, survival remains poor for patients with metastatic disease (3% at 5-year), who represent the majority (53%) of pancreatic cancer patients at the time of diagnosis (4).
In a significant percentage of cases, even in the absence of metastases, surgery cannot proceed because of the presence of vascular involvement (5). In these patients affected by locally advanced pancreatic cancer (LAPC), neoadjuvant therapy is justified by the possibility of increasing the rates of radical surgery with negative resection margins (R0) in the case of borderline resectable tumours, while in case of unresectable pancreatic cancer the therapeutic goal is to bring the patients to surgery and increase OS. The neoadjuvant treatment also favours a better selection of patients able to receive a surgical resection, and avoids it for those with a biologically more aggressive disease who progress during treatment (6).
Currently, the combination of chemotherapy and radiotherapy is the considered strategy for radical intent in patients with unresectable LAPC, or as neoadjuvant setting in borderline resectable disease (7).
Rationale and knowledge gap
An accurate and complete staging is therefore crucial for the therapeutic strategy in patients with pancreatic cancer. Our intent is to define the state of the art of the pre-treatment evaluation of pancreatic cancer and, based on the most up-to-date information in the literature, propose an evidence-based algorithm to support clinicians.
Objective
The focus of this review is to provide insight in the advances in pre-treatment evaluation of pancreatic cancer and support further strategies in the management of this disease. This review asks the question of whether integrating traditional imaging, functional imaging and minimally invasive surgical procedures before treatment may detect a complete staging for patients with pancreatic cancer, allowing the most appropriate course of treatment to be identified for them. We present the following article in accordance with the Narrative Review reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1034/rc).
Methods
Table 1 shows the search strategy summary. In this narrative review, the PubMed database was checked between 27 March 2021 and 16 January 2022. The focused keywords were “pancreatic cancer”, “imaging”, “computed tomography”, “magnetic resonance imaging”, “endoscopic ultrasonography”, “endoscopic retrograde cholangiopancreatography”, “positron emission tomography/computed tomography”, “laparoscopy”. The reference lists of relevant articles were manually searched. We used articles written in English only, published in the period between January 2000 and January 2022. The entire text of the articles, including prospective observational studies, retrospective analyses and meta-analyses, was reviewed and analysed.
Table 1
| Items | Description |
|---|---|
| Date of search | Between 27 March 2021 and 16 January 2022 |
| Databases and other sources searched | PubMed |
| Search terms used | Pancreatic cancer, imaging, computed tomography, magnetic resonance imaging, endoscopic ultrasonography, endoscopic retrograde cholangiopancreatography, positron emission tomography/computed tomography, laparoscopy |
| Timeframe | Between 3 January 2000 and 16 January 2022 |
| Inclusion, and exclusion criteria | Inclusion criteria: only articles written in English were included. Prospective observational studies, retrospective analyses and meta-analyses were reviewed and included. Exclusion criteria: not applicable |
| Selection process | Eligible articles were screened by authors GMP, PT and AC. Consensus was reached with discussion among all author |
Imaging assessment
The progressive introduction of more refined surgical techniques (e.g., difficult vascular reconstruction) and the use of preoperative and postoperative therapies over the last decade lead to the development of resectable and borderline resectable disease criteria, amplifying the request for more meticulous and specific radiological assessment of disease extent.
Crucial factors to determine the resectability of the tumour include the identification of distant metastases and vascular involvement, particularly the celiac axis (CA), superior mesenteric artery (SMA), superior mesenteric vein (SMV), and portal vein (PV) (8).
Radiologic evidence of <180° tumour interface to SMA is the most frequently used criterion for describing borderline resectable pancreatic cancer (9).
On the other hand, radiological criteria for SMV-PV involvement still lacks consensus: the American Hepatopancreatobiliary Association (AHPBA), the Society of Surgical Oncology (SSO) and the Society for Surgery of the Alimentary Tract (SSAT) consider any degree of SMV-PV abutment condition for borderline resectable cancer.
MD Anderson Cancer Centre classifies the occurrence of venous occlusion as a characteristic of borderline resectable cancer, but tumour abutment (≤180°) or encasement (>180°) of SMV-PV as resectable cancer (8).
Regardless of the imaging modality used, pancreatic cancer staging should evaluate:
- Tumour size, extension of tumour beyond the pancreas, including contiguous vasculature (i.e., SMA, CA, common hepatic artery and splenic artery, hepatic arterial variants, and the main PV, splenic vein, SMV, and whether the tumour is spreading to divisions of these veins, excluding placement of a graft).
- Presence of regional adenopathy (especially nodes outside the surgical field suspicious, based on size or morphology).
- Metastatic involvement of the liver, peritoneum, and lungs (8).
Computed tomography (CT)
Multidetector CT is the most widely used imaging modality to stage pancreatic cancer (8).
The acquisition protocol involves a biphasic examination (10):
- The pancreatic parenchymal phase (usually 45–50 seconds after the administration of contrast media, depending on injection rate), with maximal pancreatic parenchymal enhancement, ensuring best visualization of the usually hypoattenuating tumour. Moreover, the peripancreatic arteries involvement can be assessed as they are typically well opacified during this phase.
- The portal venous phase, which has a pivotal role in the detection of porto-mesenteric system and liver involvement; in this phase (acquired typically 70 seconds after the start of contrast addition) the liver is maximally enhanced, improving detection of hypodense hepatic metastases.
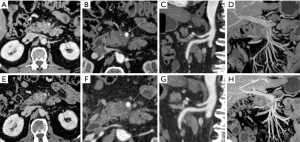
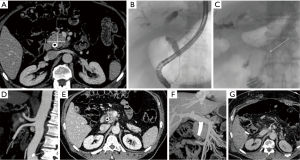
Figure 1A-1H and Figure 2A-2G represent examples of imaging of pancreatic lesions.
Magnetic resonance imaging (MRI)
MRI has been reported to have a sensitivity of 93% and specificity of 50% to 75% for determining resectability, and studies comparing state-of-the-art CT with state-of-the-art MRI report CT sensitivity of 87%, and specificity of 63% to 75%) (8).
Imaging protocol consists of the following sequences: T1-weighted in-and-out of phase gradient-echo; T2-weighted fast spin-echo; T2-weighted fat-suppressed fast spin-echo; diffusion weighted imaging (DWI); 3D T1-weighted fat-suppressed gradient echo dynamic images, including precontrast, pancreatic, venous, and equilibrium phases and T2-weighted magnetic resonance cholangiopancreatography (MRCP) (11).
MRCP can non-invasively display abnormalities of the entire pancreatic and bile duct, e.g., anatomic variations and obstructive dilatation. Furthermore, MRI, especially DWI, has been found to depict small liver metastases invisible with standard CT in approximately 10% of patients, with a subsequent change in management (12-14).
Endoscopic ultrasonography (EUS)
In the last few years, EUS has become one of the main techniques in the diagnosis of pancreatic cancer (15). It provides detailed sonographic images achieved by passing an endoscope with an ultrasound transducer at its tip into the gastrointestinal tract (16).
Although various types of ultrasound endoscopes have been developed, the radial type and the linear type are the most commonly used. The radial echoendoscope provides 360° circumferential images in a plane perpendicular to the major axis of the endoscope (similar to the images provided by a CT scan); the linear echoendoscope instead provides images on a plane parallel to the long axis of the instrument. Furthermore, the linear type allows to perform needle sampling of the pancreatic lesion (17).
Regarding the staging of PDAC, EUS has demonstrated in several studies to be superior to the CT or MRI for T staging, especially for its capacity to avoid overstaying (18).
In a recent paper written by Ikemoto and colleagues, the sensitivity of EUS for detecting small PDAC was 82%, in spite of the 58% and 38% demonstrated by the CT and MRI respectively (19).
Otherwise, no significant difference has been found in N staging between EUS and CT. N staging capacity of EUS has been assessed in a range from 64% to 82%, with higher sensitivity for peripancreatic and periceliac lymphadenopathy detection (20,21).
In addition, EUS could investigate the vascular invasion of the tumour. During the procedure, the assessment of vascular invasion in the portal system had a sensitivity of 95%, comparable with angiography and CT, with a sensitivity of 85% and 75% respectively. Lower sensitivity was shown in detecting invasion of mesenteric artery (17%) and the celiac artery (50%) (15).
However, in a prospective study conducted by Tellez-Avila et al., the accuracy of linear-EUS and CT to define vascular invasion was investigated in 50 patients with pancreatic cancer (22). EUS demonstrated to be a valid option for detecting vascular invasion, in particular the presence of arterial invasion with a 100% of predictive positive value (PPV) despite a PPV of 60% in CT (22). The limitation of EUS technique consists in the distance penetration of the ultrasound waves. Echoendoscopes provide an accurate view of surrounding structures up to approximately 5–6 cm from the instrument (23).
In addition to the possibility to detect and investigate the morphology of the pancreatic lesions, EUS could also provide histological sampling to achieve a definite diagnosis.
More in detail, specific designed needles are introduced into the ultrasound endoscope and, under direct ultrasound guidance, can cross the wall of the duodenum or the stomach and perform the biopsy avoiding damage to the surrounding vascular structures. The sample can consist both in a cytology specimen (called fine needle aspiration, FNA) or in a fine core of tissue (called fine needle biopsy, FNB) (17).
The size of the needles varies from 19 to 25 gauge (GI). In a meta-analysis published in 2016, Bang et al. showed how 25 G needles had a mayor sensitivity and specificity (93% and 97% respectively) when compared with the 22 G needle (85% sensitivity and 99% specificity) in the detection of PDAC (24).
Over the last few years, EUS-FNA has proven its effectiveness in detection of pancreatic cancer. As demonstrated by several published series, EUS has a sensitivity greater than 90% for detection of PDAC (19,25).
More in detail, EUS-FNA showed a sensitivity of up to 99% (versus 55% demonstrated by CT) for lesions with a diameter between 2 and 3 cm (26,27).
In addition to high sensitivity, EUS-FNA showed a high negative predictive value, acquiring even more value in detection of PDAC (15).
Until a few years ago, EUS-FNA was considered the standard procedure for the diagnosis of pancreatic cancer. The role of the EUS-FNB was limited to those cases in which the sample obtained with the EUS-FNA was not diagnostic due to the small amount of tissue collected.
In a multicentre randomized clinical trial, Becker et al. demonstrated an increased accuracy of the FNB over the FNA for the diagnosis of pancreatic masses (91.4% of accuracy in FNB samples compared to 80% in FNA samples) (28).
Moreover, in patients with chronic pancreatitis the inflammatory alteration of the parenchyma with calcifications inside, can limit the quality of the images and the sample of the tissue. In these cases, the FNB may be more useful and effective in diagnosing PDAC (23,29).
As reported by Yousaf et al. the EUS-FNB significantly reduces both procedural time and hospitalization with no increased risks for patients. The need for fewer passages and not needing more complex methods reduces the costs without decreasing the quality of the sample and the diagnostic power (30).
The overall complication rate after EUS-FNA procedure is significantly reduced when compared to any other modalities with a range between 1.1% and 3.8% (31).
The risk of acute pancreatitis after EUS is significantly lower as demonstrated by Eloubeidi et al. when compared to the endoscopic retrograde cholangiopancreatography (ERCP) brushing or the percutaneous biopsy. The pancreatitis risk after EUS ranges from 0.3% to 0.9%, compared to a higher rate in the ERCP (up to 21%) and percutaneous biopsies (4%) (32).
In a systematic review published by Wang et al. in 2011, the authors showed how the rates of common complications of endoscopic procedures were not significantly high. More in detail, the Authors reported that the rates of post-procedural bleeding, pain, fever and infection after EUS-FNA were 0.38%, 0.10%, 0.08% and 0.02%, respectively (33).
Even considering the malignant peritoneal seeding after procedure, the EUS-FNA had shown significantly lower rates of such important complication (2.2% vs. 16.3% in the percutaneous biopsy) (34).
Currently, EUS plays an important role in the determination of the correct stage of pancreatic cancers by providing cytological and histological confirmation.
In a trial published in 2013, Bang et al. showed that the fanning technique, consisting of multiple biopsies from different sites of the tumour, was superior to the standard technique in the diagnosis of PDAC (35).
Recently, the immediate on-site cytopathology evaluation (OCE) has been studied. OCE is a pathological procedure that allows to maximize the ability of EUS-FNA and could provide immediate feedback concerning the content and adequacy of a specimen for an accurate diagnosis with the minimum number of passes. In addition, OCE could also determine if the specimen quantity is enough for a specific test, such as immunohistochemistry. A prospective multicentre randomized controlled trial conducted by Wani and colleagues in 2015 reported the strength of the OCE in reducing the number of needle passes (OCE = 4 vs. no OCE = 7; P<0.0001) without increasing the duration time of the procedure, the complications and the costs of the EUS (36).
However, Lee et al. in a multicentre randomized study demonstrated the non-inferiority of the traditional biopsy with 7 needle passes over the OCE with significant lower costs (37).
Its ability to provide morphological and cytological information, combined with the low risk of peri-procedural complications, are making EUS a key method in the early diagnosis of pancreatic cancer. However, the fields of application of the EUS are not limited only to the diagnosis of PADC, but it is also making its way into other aspects in the management of patients with pancreatic cancer, such as decompression of the biliary tract.
ERCP
In this technique, endoscopy is combined with fluoroscopy allowing a detailed study of the pancreatobiliary ductal systems. The development of new diagnostic techniques, such as EUS, and innovations in diagnostic imaging, such as cholangioMR, combined with the overall risk of severe complications after ERCP, had progressively decreased its diagnostic role. However, ERCP still maintains an important role in the management of pancreatobiliary diseases (38).
To justify the risk of the procedure, ERCP is currently generally performed with two indications: tumour sampling or stenting, and decompression of the biliary tree.
There are different ways to collect the tumour sample in ERCP. Firstly, the biliary brushing: with the ERCP brushing the sample is obtained by an 8 French (Fr) brush introduced through a catheter using a guidewire. In a prospective series of 1,285 patients published in 2018, Moura et al. showed that the sensitivity of the brushing of the bile duct ranged between 30% to 78% (median 54%), with a specificity of 97% to 100% (median 100%) (39).
Cytological brushing represents the safest procedure with minimal risk of complications, such as pancreatitis and perforation of the bile duct (38).
Taking into consideration the low sensitivity of brushing, new biopsy methods in ERCP have been developed. Among these methods, there is the fluoroscopic guided biopsy. In this technique, deeper tissue samples are obtained with respect to the epithelial layer of the brushing. It is routinely performed by introducing forceps (between 5 and 10 Fr) in the endoscopic instrument. Over the last years, some authors demonstrated that endobiliary forceps biopsy has a sensitivity range of 36–81% (median 61%), and a specificity of 90% to 100% (median 100%) for the diagnosis of malignant biliary strictures. These characteristics cause a low negative predictive value of 58% (40-42).
In order to increase the sensitivity of the ERCP sample tissue, Ponchon and colleagues published a study showing that the combination of both brushings and forceps biopsies can increase the diagnostic yield to a sensitivity of 63% to 86% and a specificity of 97% to 100% (43).
In 2000, Jailwala et al. confirmed those findings and also highlighted that the results obtained remained suboptimal compared to the EUS standards (44). Navaneethan and colleagues described the cholangioscopy-guided biopsy, which is a technique performed by introducing a cholangioscope through a duodenoscope, allowing the direct visualization of the biliary stricture, and permitting the direct visualization of intraductal nodules or the presence of papillary or villous mucosal projections that represents the main features of PDAC in cholangioscopic technique (40).
The cholangioscopic biopsy had a sensitivity range from 88% to 100%, and specificity from 77% to 92% in the diagnosis of pancreatobiliary malignancy (45).
These results, however, should be reconsidered due to the higher risk of complications despite a standard ERCP. More in detail, complications include pancreatitis, bile duct perforation, haemorrhage, air embolization, and cholangitis. Taking these concerns into consideration, the use of cholangioscopy is limited for selected cases of unapproachable ductal lesions (45).Another application of the ERCP is the ERCP-guided naso-pancreatic drainage (ENPD), described for the first time in 1974. This technique foresees a pancreatic juice collection 2 to 6 times a day bile sampling collected for up to 3 days. This method had shown an 80% sensitivity, 100% specificity, 100% positive predictive value, 71% negative predictive value, and 87% overall accuracy (46).
Hanada and colleagues in a recent study published in 2019, suggested a role for the ERCP-guided serial pancreatic juice aspiration cytologic examination (SPACE) in diagnosis of pancreatic cancers smaller than 1 cm (47).
To date, ERCP-guided biliary sampling is indicated only in cases of an unresectable tumour which requires biliary system decompression (48).
In general, EUS had shown its overall superiority compared to the ERCP with a sensitivity of 43–94% (median 81%) vs. 13–81% (median 52%) and specificity of 93–100% (median 100%) vs. 75–100% (median 100%) (49).
In 2016, Malak et al. confirmed those results in a retrospective study of 234 patients with PDAC. They demonstrated the advantage of EUS compared to the ERCP, with an overall adverse event significantly lower for EUS-FNA (1.9% vs. 6.6%) (50).
Moreover, ERCP is associated with high rates of complications as post-ERCP cholangitis, pancreatitis, cholecystitis, liver abscess, biliary ductal perforation, haemorrhage, stent migration or obstruction (51).
To date, ERCP can be considered in patients who are non resectable PDAC, who are candidates for first line chemotherapy and/or chemoradiation therapy, or in those patients with a malignant biliary duct obstruction who need to be treated with a neoadjuvant therapy before surgery (52,53).
The role of ERCP and biliary stenting in resectable PDAC with obstructive jaundice is debated and controversial. Even if the grade of biliary obstruction is associated with higher rates of post-operative morbidities, as reported by many studies, there is no scientific evidence recommending ERCP before upfront surgery, even with high levels of bilirubinaemia (54).
Moreover, as demonstrated by many authors over the last decade, preoperatory ERCP is associated with bacterobilia, which plays a fundamental role in determining post-operative infectious complications after pancreatoduodenectomy (55).
In addition, some authors referred to the role of bacterobilia in the development and severity of pancreatic fistula (56).
In conclusion, as reported by Nakai et al. in a recent study, the decision to submit a patient with a resectable PDAC to an endoscopic stenting procedure must be discussed in a multidisciplinary meeting to optimize every decision singularly (48).
In considering all those findings, ERCP can still be considered a fundamental source in the management of pancreatic cancer, but with specific indications.
Currently, ERCP has a wide range of use in patients with borderline resectable, locally advanced and not resectable PDAC, who are candidates for first line chemotherapy with neoadjuvant or palliative intent (52,53).
As previously reported, currently the role of the ERCP in the early diagnosis of pancreatic cancer is not considered to be at the same level as other procedures with lower rates of post-procedural complications. However, the role of ERCP in the stenting of the biliary tree is increasing due to the more advanced medical and surgical strategies often proposed to patients with pancreatic adenocarcinoma. The failure of the stenting could be the cause of patients dropping out of their therapeutic protocols.
Positron emission tomography/computed tomography (PET/CT)
18F-Fluorodeoxyglucose (18F-FDG) PET/CT plays an additional role in identifying distant metastases and in assessing response to treatment. It allows detection of the presence of metastatic tumour cells, which sometimes are not detectable by other imaging methods both at the lymph nodes level and at distance, particularly in the liver and peritoneum, even in patients with resectable pancreatic cancer. In 2009, Kauhanen et al. referred 38 patients with suspected pancreatic neoplasia to 18F-FDG PET-CT, CT, and MRI. Seventeen tumours with pancreatic adenocarcinoma histology, 3 neuroendocrine tumours, 4 pancreatitis, 6 cystic lesions, and 2 fibrotic lesions were diagnosed. The accuracy of PET-CT for the diagnosis of pancreatic neoplasia was 89%, compared with 76% on CT and 79% on MRI. Regarding sensitivity in detecting lymph nodes and distant metastases, the result was 30% and 88% with PET-CT compared with 30% and 38% with CT and MR (57).
In 2013, Asagi et al. observed that among 149 patients with pancreatic cancer who underwent 18F-FDG PET-CT, the accuracy was 80% in assessing local invasion, 94% in detecting distant metastases, and only 42% in detecting lymph node metastases (58).
In a paper by Crippa et al., 18F-FDG PET-CT was performed in 72 patients with resectable pancreatic cancer. In 11% of patients, PET-CT diagnosed the presence of distant metastases, thus avoiding unwarranted surgery. PET-CT showed a sensitivity and specificity in detecting distant metastases of 78% and 100%, respectively (59).
In 2015, Burge et al. in a prospective single-center study, 56 patients with potentially operable neoplasia of the pancreas, distal bile ducts, and ampulla underwent accurate presurgical imaging, including PET-CT. In nine cases (16%) the surgical option was abandoned because of the detection of distant metastases on PET-CT. In four patients, metastases were not detected by PET-CT, and seven patients were inoperable because of the presence of vascular invasion (60).
In addition, metabolic parameters derived from pre-treatment 18F-FDG PET/CT have shown to play a prognostic and predictive role for patients with pancreatic cancer.
Piemento et al. evaluated 105 patients with stage I-II pancreatic cancer who had performed preoperative PET-CT. Fifty-one patients had low uptake (SUVmax <5.5) and 54 patients had high uptake (SUVmax >5.5). Patients with low SUVmax had a higher median OS than patients with high SUVmax (28 vs. 16 months; P=0.036), as well as a better PFS (14 vs. 12 months; P=0.049) (61).
In 2015, Wang et al. aimed to evaluate SUVmax as a prognostic marker for patients with LAPC. Sixty-nine patients were enrolled. A high SUVmax value (>5.5) was observed in thirty-five patients who had significantly worse OS and PFS than patients with low SUVmax (<5.5) (P=0.025 and P=0.003, respectively) (62).
PET-CT can predict the possible efficacy of neoadjuvant treatment by assessing volumetric parameters. Sakane et al. studied 25 patients who underwent gemcitabine-based neoadjuvant chemoradiotherapy. The Evans grading system was used to assess response, and volumetric parameters such as SULpeak (uptake value corrected for the patient’s lean body mass), MTV (metabolic tumour volume), TLG (total lesion glycolysis) of the baseline PET and re-evaluation PET were compared. Eight patients (32%) showed a poor response (Evans grade I), eleven patients (44%) showed a mild response (Evans grade IIa), and six patients (24%) had a moderate response (Evans grade IIb). Of these, six patients (24%) were assigned to the responders group because they had a response with more than 50% reduction in tumour cells, and the remaining 19 (76%) were assigned to the non-responders group. Parameter analysis showed that in patients with a high post-treatment SULpeak and a positive MTV/TLG ratio, an unfavourable effect on histopathological response to chemoradiotherapy can be predicted (63).
Fiore et al. evaluated the predictive value of 18F-FDG PET/CT semiquantitative parameters of the primary tumour and CA 19-9 levels evaluated before treatment in 58 patients with LAPC. Pre-treatment CA 19-9 level, as well as MTV and TLG values of primary tumour at baseline 18F-FDG PET/CT and their combination were significant predictors of early progression (EP), local progression (LP) and OS (64).
The advantage in the detection of lymph nodes and distant metastases has also been demonstrated in the assessment of response after neoadjuvant treatments. Wartski et al. in a 2019 review positively evaluated the performance of 18F-FDG PET-CT in detecting affected lymph nodes and distant metastases, in both initial staging and reassessment after induction treatments (65).PET-CT can detect response after neoadjuvant treatment by a reduction in SUV that has shown significant changes. Sometimes the reduction in SUV correlates with a reduction in serum Ca 19.9 levels (66).
A meta-analysis by Wang et al. included 23 studies with a total of 1,762 patients in which the correlation of PET-CT parameters (SUVmax, MTV, TLG) with the prognosis of patients with pancreatic cancer was evaluated. The results showed that a high SUVmax value of the primary tumor correlated with a poor prognosis [hazard ratio (HR) 1.31; 95% confidence interval (CI): 1.15–1.5; P<0.001]. However, a reduction in SUVmax value after active treatments indicated better OS than in patients without a reduction in SUVmax (HR 0.68; 95% CI: 0.47–0.98; P=0.037) (67).
In 2020, Sperti et al. subjected 144 patients with resectable pancreatic cancer to 18F-FDG PET-CT, dividing them into two groups according to SUVmax value: 82 patients with high uptake (SUVmax >3.65) and 62 patients with low uptake (SUVmax <3.65). Patients with low uptake showed better OS than patients with high uptake (P<0.001), demonstrating that SUVmax is an important prognostic factor and can be used in the management of patients with pancreatic cancer (68).
Barnes et al. evaluated the prognostic value of PET-CT in patients with localized pancreatic cancer undergoing neoadjuvant treatment. In this cohort of patients with pancreatic carcinoma, pretreatment CA 19-9 and SUVmax values were also prognostic markers (69).
Zimmermann et al. in a prospective single-center phase II study aimed to evaluate the prognostic value of 18F-FDG PET-CT and diffusion-enhanced MRI (DW-MRI) before and after neoadjuvant chemoradiotherapy. Enrolled patients had resectable, borderline resectable, and unresectable pancreatic cancer without evidence of distant metastasis. Patients underwent induction chemotherapy with gemcitabine and oxaliplatin for 2 cycles and subsequent chemoradiation with weekly gemcitabine. A total of 25 patients were enrolled. The response rate detected by 18F-FDG PET-CT was 85% with a statistically significant reduction in SUVmax after treatment. Using the apparent diffusion coefficient (ADC) of DW-MRI such treatment responses are not detectable. After neoadjuvant treatment, 16 patients underwent surgery, of whom 12 underwent tumour resection with negative surgical resection margins (70).
In addition to the above, PET-CT is critical in determining the volumes to be irradiated at the time of treatment planning as it improves delineation of tumour margins when compared to CT alone. The use of PET-CT in pancreatic cancer may lead to automation in the process of target contouring and in the identification of areas on which a dose boost could be performed, allowing a reduction in target volume and therefore a greater sparing of organs at risk with greater safety in the execution of the dose boost (71).
Staging laparoscopy
Exploratory or diagnostic laparoscopy (terms used to distinguish it from operative or therapeutic laparoscopy) is a minimally invasive surgical technique, thanks to which it is possible to access the abdominal and pelvic cavity of a patient, without resorting to the large incisions required by traditional open surgery. In approximately 8% of patients with pancreatic cancer, there is the presence of occult abdominal metastases, undetectable by imaging techniques. Therefore, in those patients presenting with high serum CA 19-9 levels, increased tumour size and description of lesions of undetermined nature on CT, it may be necessary to resort to exploratory laparoscopy (72).CT has moderate sensitivity (65–88%) and specificity (38–63%) in detecting peritoneal metastases. At the time of staging, peritoneal metastases can be detected by the use of exploratory laparoscopy in more than 7% of patients with locally advanced pancreatic neoplasia (73).
In 2016, Karabicak et al. evaluated 110 patients with LAPC who underwent exploratory staging laparoscopy with the aim of excluding distant metastases. Occult distant metastases were detected in 62 patients (56.4%), specifically peritoneal washing was positive in 23% of cases, peritoneal carcinosis in 19% and liver metastases in 15%. On multivariate analysis, CA 19-9 values >60 U/mL and tumor size >55 mm were found as risk factors for latent metastases. According to the authors of this work in this group of patients exploratory laparoscopy should be routinely used as a staging examination (74).
Satoi et al. considered the role of laparoscopy for patient selection and prognostic factors in patients with LAPC. Sixty-seven patients were evaluated and divided into four groups according to the site of metastases: group I: 16 patients with positive peritoneal washing without distant metastasis; group II: 13 patients with peritoneal dissemination; group III: 10 patients with liver metastasis; and group IV: 28 patients with negative peritoneal washing and no distant metastasis. Most patients (39 patients, 58.2%) had occult metastases. The median survival was 13 months in group I, 11 months in group IV, and 7 months in groups II and III (P<0.05) (75).
A systematic review by De Rosa et al., aimed at identifying indications for performing staging laparoscopy in patients with resectable pancreatic cancer, evaluated 24 studies. It was found that factors for selecting patients for laparoscopy to predict unresectability included CA 19-9 values >150 U/mL and tumor size >3 cm (76).
In 2016, Levy et al. reviewed the use of diagnostic laparoscopy with ultrasound (DLUS) to determine the resectability of pancreatic tumours compared with standard imaging represented by CT. A total of 104 studies were identified, including 19 prospective studies, with a total of 1,573 patients. The use of DLUS correctly predicted resectability status in 79% of cases compared with 55% with standard imaging. The situations that precluded resectability in most cases were of liver metastases, vascular involvement, and peritoneal metastases. The use of DLUS allowed a reduction in the performance of noncurative interventions (77). Yamura et al. evaluated the prognostic impact of staging laparoscopy, compared with exploratory laparotomy, in evaluating resectability in pancreatic cancer in the preoperative phase. A total of 195 patients with resectable pancreatic neoplasia were evaluated, of whom 57 underwent exploratory laparoscopy (Group I), while 138 underwent laparotomy directly (Group II). In the first group, there were 20 patients (35%) in whom it was not possible to proceed with surgery due to the presence of vascular involvement or distant metastases, in the second group there were eight (11%). According to this work, laparoscopy prevents the performance of unnecessary laparotomies (78). A meta-analysis by Ta et al. analyzed the use of staging laparoscopy in patients with resectable and borderline resectable pancreatic cancer. Fifteen studies were included, with 2,776 patients meeting the inclusion criteria. In 12 studies, of 1,756 patients with resectable pancreatic cancer after staging with CT and MRI, 350 cases (20%) of unresectable neoplasia were detected through laparoscopy. In three studies, among 242 patients with LAPC, staging laparoscopy identified metastases in 86 patients (36%). The failure rate of exploratory laparoscopy to detect unresectable tumors was 5% (64 of 1,406). Thus, laparoscopy is essential both in avoiding non-therapeutic surgery and, in LAPC, for accurate selection of patients for neoadjuvant treatment (79).
Limitations
Our review has some limitations. The quality of the evidence was limited in several studies, with few phase III trials. It was difficult to aggregate all modalities of imaging and have a direct comparison between them. For this reason, we decided to adopt the style of narrative review in this paper. Despite these limitations, this review provides the most reliable data reported in literature and highlights an unmet clinical need to improve our understanding of the integration of traditional imaging, functional imaging and minimally invasive surgical procedures before treatment.
Conclusions
An accurate staging of PDAC is challenging for its aggressive biological behavior, frequently associated with extra-pancreatic dissemination to lymph nodes and distant organs, which may be occult or difficult to identify by single imaging technique. The effort of the pre-treatment evaluation should be to identify an overt or potentially occult systemic disease, and the possibility of a complete surgical resection. Each imaging modality has its own diagnostic advantages and limitations. The sensitivity, specificity and accuracy for each image set are shown in Table 2. A multimodal pre-treatment workup should be searched as it improves staging accuracy, orienting patients with resectable PDAC towards surgical resection, optimizing patient selection with LAPC to neoadjuvant or definite therapy (chemotherapy and radiotherapy) and avoiding surgery or curative radiation therapy in those with metastatic disease (Figure 3).
Table 2
| CT | MRI | PET-CT | Laparoscopy | ERCP | EUS | |
|---|---|---|---|---|---|---|
| Tumor detection (2–3 cm in size) | ||||||
| Ikemoto et al. (19) | ||||||
| Specificity | – | – | – | – | – | – |
| Sensitivity | 58% | 38% | – | – | 84% | 82% |
| Accuracy | – | – | – | – | – | – |
| Gonzalo-Marin et al. (15) | ||||||
| Specificity | – | – | – | – | – | – |
| Sensitivity | 55% | – | – | – | – | 99% |
| Accuracy | – | – | – | – | – | – |
| Ta et al. (79) | ||||||
| Specificity | 100% | – | – | 100% | – | |
| Sensitivity | 56% | – | – | 56% | – | |
| Accuracy | – | – | – | – | – | |
| Kauhanen et al. (57) | ||||||
| Specificity | 67% | 72% | 94% | – | – | |
| Sensitivity | 85% | 85% | 85% | – | – | |
| Accuracy | 76% | 79% | 89% | – | – | |
| Tumour staging | ||||||
| Kulig et al. (20) | ||||||
| Specificity | 69.2% | – | – | – | – | 84.6% |
| Sensitivity | 88.8% | – | – | – | – | 96.1% |
| Accuracy | 90.0% | – | – | – | – | 82.5% |
| Qayyum et al. (8) | ||||||
| Specificity | 63–75% | 50–75% | – | – | – | 91% |
| Sensitivity | 87% | 93% | – | – | – | 85% |
| Accuracy | – | – | – | – | – | – |
| Asagi et al. (58) | ||||||
| Specificity | – | – | – | – | – | – |
| Sensitivity | – | – | – | – | – | – |
| Accuracy | – | – | 80% | – | – | – |
| Lymphadenopathy | ||||||
| Qayyum et al. (8) | ||||||
| Specificity | – | – | 81% | – | – | 85% |
| Sensitivity | – | – | 64% | – | – | 58% |
| Accuracy | – | – | – | – | – | – |
| Crippa et al. (59) | ||||||
| Specificity | 93% | – | – | – | – | – |
| Sensitivity | 21% | – | – | – | – | – |
| Accuracy | – | – | – | – | – | – |
| Asag et al. (58) | ||||||
| Specificity | – | – | – | – | – | – |
| Sensitivity | – | – | – | – | – | – |
| Accuracy | 35% | – | 42% | – | – | – |
| Liver metastasis | ||||||
| Qayyum et al. (8) | ||||||
| Specificity | – | – | 96% | – | – | – |
| Sensitivity | 70–76% | 90–100% | 67% | – | – | – |
| Accuracy | – | – | – | – | – | – |
| Kim et al. (13) | ||||||
| Specificity | – | – | – | – | – | – |
| Sensitivity | – | GADOXETIC 92.5–93.8% | – | – | – | – |
| FERUCARB–OTRAN 87.5–88.8% | ||||||
| Accuracy | – | – | – | – | – | – |
| Crippa et al. (59) | ||||||
| Specificity | – | – | 100% | – | – | – |
| Sensitivity | – | – | 78% | – | – | – |
| Accuracy | – | – | – | – | – | – |
CT, computed tomography; MRI, magnetic resonance imaging; PET-CT, positron emission tomography-computed tomography; laparoscopy; ERCP, endoscopic retrograde cholangiopancreatography; EUS, endoscopic ultrasonography.
In Figure 4, we propose an evidence-based multimodal pre-treatment algorithm for PDAC staging.
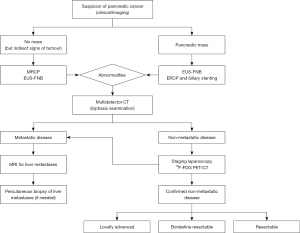
The selection of patients affected by PDAC remains a decisive matter in the debate on integrated treatments. In our opinion, the diagnostic workup protocol should combine imaging exams with laparoscopy to better select patients for chemotherapy and radiotherapy, as well as for their selection for surgery.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Oncology for the series “Pancreas Surgery”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1034/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1034/coif). The series “Pancreas Surgery” was commissioned by the editorial office without any funding or sponsorship. AC served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work, including ensuring that any questions related to the accuracy or integrity of any part of the work have been appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [Crossref] [PubMed]
- Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913-21. [Crossref] [PubMed]
- McGuigan A, Kelly P, Turkington RC, et al. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol 2018;24:4846-61. [Crossref] [PubMed]
- National Cancer Institute. Cancer Stat Facts: Pancreatic Cancer. Surveillance, Epidemiology and End Results Program. 2019.
- Vincent A, Herman J, Schulick R, et al. Pancreatic cancer. Lancet 2011;378:607-20. [Crossref] [PubMed]
- Fiore M, Ramella S, Valeri S, et al. Phase II study of induction chemotherapy followed by chemoradiotherapy in patients with borderline resectable and unresectable locally advanced pancreatic cancer. Sci Rep 2017;7:45845. [Crossref] [PubMed]
- Silvestris N, Brunetti O, Bittoni A, et al. Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up of Exocrine Pancreatic Ductal Adenocarcinoma: Evidence Evaluation and Recommendations by the Italian Association of Medical Oncology (AIOM). Cancers (Basel) 2020;12:1681. [Crossref] [PubMed]
- Qayyum A, Tamm EP, et al. ACR Appropriateness Criteria(®) Staging of Pancreatic Ductal Adenocarcinoma. J Am Coll Radiol 2017;14:S560-9. [Crossref] [PubMed]
- Mollberg N, Rahbari NN, Koch M, et al. Arterial resection during pancreatectomy for pancreatic cancer: a systematic review and meta-analysis. Ann Surg 2011;254:882-93. [Crossref] [PubMed]
- Al-Hawary MM, Francis IR, Chari ST, et al. Pancreatic ductal adenocarcinoma radiology reporting template: consensus statement of the society of abdominal radiology and the american pancreatic association. Gastroenterology 2014;146:291-304.e1. [Crossref] [PubMed]
- Rhee H, Park MS. The Role of Imaging in Current Treatment Strategies for Pancreatic Adenocarcinoma. Korean J Radiol 2021;22:23-40. [Crossref] [PubMed]
- Motosugi U, Ichikawa T, Morisaka H, et al. Detection of pancreatic carcinoma and liver metastases with gadoxetic acid-enhanced MR imaging: comparison with contrast-enhanced multi-detector row CT. Radiology 2011;260:446-53. [Crossref] [PubMed]
- Kim YK, Lee YH, Kwak HS, et al. Detection of liver metastases: Gadoxetic acid-enhanced three-dimensional MR imaging versus ferucarbotran-enhanced MR imaging. Eur J Radiol 2010;73:131-6. [Crossref] [PubMed]
- Sheridan MB, Ward J, Guthrie JA, et al. Dynamic contrast-enhanced MR imaging and dual-phase helical CT in the preoperative assessment of suspected pancreatic cancer: a comparative study with receiver operating characteristic analysis. AJR Am J Roentgenol 1999;173:583-90. [Crossref] [PubMed]
- Gonzalo-Marin J, Vila JJ, Perez-Miranda M. Role of endoscopic ultrasound in the diagnosis of pancreatic cancer. World J Gastrointest Oncol 2014;6:360-8. [Crossref] [PubMed]
- Singh A, Faulx AL. Endoscopic Evaluation in the Workup of Pancreatic Cancer. Surg Clin North Am 2016;96:1257-70. [Crossref] [PubMed]
- Zar S, Kohoutová D, Bureš J. Pancreatic Adenocarcinoma: Epidemiology, Role of EUS in Diagnosis, Role of ERCP, Endoscopic Palliation. Acta Medica (Hradec Kralove) 2019;62:131-6. [Crossref] [PubMed]
- Soriano A, Castells A, Ayuso C, et al. Preoperative staging and tumor resectability assessment of pancreatic cancer: prospective study comparing endoscopic ultrasonography, helical computed tomography, magnetic resonance imaging, and angiography. Am J Gastroenterol 2004;99:492-501. [Crossref] [PubMed]
- Ikemoto J, Serikawa M, Hanada K, et al. Clinical Analysis of Early-Stage Pancreatic Cancer and Proposal for a New Diagnostic Algorithm: A Multicenter Observational Study. Diagnostics (Basel) 2021;11:287. [Crossref] [PubMed]
- Kulig J, Popiela T, Zajac A, et al. The value of imaging techniques in the staging of pancreatic cancer. Surg Endosc 2005;19:361-5. [Crossref] [PubMed]
- Yasuda K, Mukai H, Nakajima M, et al. Staging of pancreatic carcinoma by endoscopic ultrasonography. Endoscopy 1993;25:151-5. [Crossref] [PubMed]
- Tellez-Avila FI, Chavez-Tapia NC, López-Arce G, et al. Vascular invasion in pancreatic cancer: predictive values for endoscopic ultrasound and computed tomography imaging. Pancreas 2012;41:636-8. [Crossref] [PubMed]
- Fusaroli P, Spada A, Mancino MG, et al. Contrast harmonic echo-endoscopic ultrasound improves accuracy in diagnosis of solid pancreatic masses. Clin Gastroenterol Hepatol 2010;8:629-34.e1-2.
- Bang JY, Hawes R, Varadarajulu S. A meta-analysis comparing ProCore and standard fine-needle aspiration needles for endoscopic ultrasound-guided tissue acquisition. Endoscopy 2016;48:339-49. [PubMed]
- Rösch T, Lorenz R, Braig C, et al. Endoscopic ultrasound in pancreatic tumor diagnosis. Gastrointest Endosc 1991;37:347-52. [Crossref] [PubMed]
- Luz LP, Al-Haddad MA, Sey MS, et al. Applications of endoscopic ultrasound in pancreatic cancer. World J Gastroenterol 2014;20:7808-18. [Crossref] [PubMed]
- Owens DJ, Savides TJ. Endoscopic ultrasound staging and novel therapeutics for pancreatic cancer. Surg Oncol Clin N Am 2010;19:255-66. [Crossref] [PubMed]
- Becker D, Strobel D, Bernatik T, et al. Echo-enhanced color- and power-Doppler EUS for the discrimination between focal pancreatitis and pancreatic carcinoma. Gastrointest Endosc 2001;53:784-9. [Crossref] [PubMed]
- van Riet PA, Larghi A, Attili F, et al. A multicenter randomized trial comparing a 25-gauge EUS fine-needle aspiration device with a 20-gauge EUS fine-needle biopsy device. Gastrointest Endosc 2019;89:329-39. [Crossref] [PubMed]
- Yousaf MN, Chaudhary FS, Ehsan A, et al. Endoscopic ultrasound (EUS) and the management of pancreatic cancer. BMJ Open Gastroenterol 2020;7:e000408. [Crossref] [PubMed]
- Raut CP, Grau AM, Staerkel GA, et al. Diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration in patients with presumed pancreatic cancer. J Gastrointest Surg 2003;7:118-28. [Crossref] [PubMed]
- Eloubeidi MA, Gress FG, Savides TJ, et al. Acute pancreatitis after EUS-guided FNA of solid pancreatic masses: a pooled analysis from EUS centers in the United States. Gastrointest Endosc 2004;60:385-9. [Crossref] [PubMed]
- Wang KX, Ben QW, Jin ZD, et al. Assessment of morbidity and mortality associated with EUS-guided FNA: a systematic review. Gastrointest Endosc 2011;73:283-90. [Crossref] [PubMed]
- Mohammad Alizadeh AH, Shahrokh S, Hadizadeh M, et al. Diagnostic potency of EUS-guided FNA for the evaluation of pancreatic mass lesions. Endosc Ultrasound 2016;5:30-4. [Crossref] [PubMed]
- Bang JY, Magee SH, Ramesh J, et al. Randomized trial comparing fanning with standard technique for endoscopic ultrasound-guided fine-needle aspiration of solid pancreatic mass lesions. Endoscopy 2013;45:445-50. [Crossref] [PubMed]
- Wani S, Mullady D, Early DS, et al. The clinical impact of immediate on-site cytopathology evaluation during endoscopic ultrasound-guided fine needle aspiration of pancreatic masses: a prospective multicenter randomized controlled trial. Am J Gastroenterol 2015;110:1429-39. [Crossref] [PubMed]
- Lee LS, Nieto J, Watson RR, et al. Randomized Noninferiority Trial Comparing Diagnostic Yield of Cytopathologist-guided versus 7 passes for EUS-FNA of Pancreatic Masses. Dig Endosc 2016;28:469-75. [Crossref] [PubMed]
- Yousaf MN, Ehsan H, Wahab A, et al. Endoscopic retrograde cholangiopancreatography guided interventions in the management of pancreatic cancer. World J Gastrointest Endosc 2020;12:323-40. [Crossref] [PubMed]
- Moura DTH, de Moura EGH, Matuguma SE, et al. EUS-FNA versus ERCP for tissue diagnosis of suspect malignant biliary strictures: a prospective comparative study. Endosc Int Open 2018;6:E769-77. [Crossref] [PubMed]
- Navaneethan U, Njei B, Lourdusamy V, et al. Comparative effectiveness of biliary brush cytology and intraductal biopsy for detection of malignant biliary strictures: a systematic review and meta-analysis. Gastrointest Endosc 2015;81:168-76. [Crossref] [PubMed]
- Pugliese V, Conio M, Nicolò G, et al. Endoscopic retrograde forceps biopsy and brush cytology of biliary strictures: a prospective study. Gastrointest Endosc 1995;42:520-6. [Crossref] [PubMed]
- Burnett AS, Calvert TJ, Chokshi RJ. Sensitivity of endoscopic retrograde cholangiopancreatography standard cytology: 10-y review of the literature. J Surg Res 2013;184:304-11. [Crossref] [PubMed]
- Ponchon T, Gagnon P, Berger F, et al. Value of endobiliary brush cytology and biopsies for the diagnosis of malignant bile duct stenosis: results of a prospective study. Gastrointest Endosc 1995;42:565-72. [Crossref] [PubMed]
- Jailwala J, Fogel EL, Sherman S, et al. Triple-tissue sampling at ERCP in malignant biliary obstruction. Gastrointest Endosc 2000;51:383-90. [Crossref] [PubMed]
- Sethi A, Chen YK, Austin GL, et al. ERCP with cholangiopancreatoscopy may be associated with higher rates of complications than ERCP alone: a single-center experience. Gastrointest Endosc 2011;73:251-6. [Crossref] [PubMed]
- Mikata R, Ishihara T, Tada M, et al. Clinical usefulness of repeated pancreatic juice cytology via endoscopic naso-pancreatic drainage tube in patients with pancreatic cancer. J Gastroenterol 2013;48:866-73. [Crossref] [PubMed]
- Hanada K, Minami T, Shimizu A, et al. Roles of ERCP in the Early Diagnosis of Pancreatic Cancer. Diagnostics (Basel) 2019;9:30. [Crossref] [PubMed]
- Nakai Y, Isayama H, Wang HP, et al. International consensus statements for endoscopic management of distal biliary stricture. J Gastroenterol Hepatol 2020;35:967-79. [Crossref] [PubMed]
- Weilert F, Bhat YM, Binmoeller KF, et al. EUS-FNA is superior to ERCP-based tissue sampling in suspected malignant biliary obstruction: results of a prospective, single-blind, comparative study. Gastrointest Endosc 2014;80:97-104. [Crossref] [PubMed]
- Malak M, Masuda D, Ogura T, et al. Yield of endoscopic ultrasound-guided fine needle aspiration and endoscopic retrograde cholangiopancreatography for solid pancreatic neoplasms. Scand J Gastroenterol 2016;51:360-7. [Crossref] [PubMed]
- Silviera ML, Seamon MJ, Porshinsky B, et al. Complications related to endoscopic retrograde cholangiopancreatography: a comprehensive clinical review. J Gastrointestin Liver Dis 2009;18:73-82. [PubMed]
- Kozarek R. Role of preoperative palliation of jaundice in pancreatic cancer. J Hepatobiliary Pancreat Sci 2013;20:567-72. [Crossref] [PubMed]
- Lee PJ, Podugu A, Wu D, et al. Preoperative biliary drainage in resectable pancreatic cancer: a systematic review and network meta-analysis. HPB (Oxford) 2018;20:477-86. [Crossref] [PubMed]
- Coté GA, Sherman S. Endoscopic palliation of pancreatic cancer. Cancer J 2012;18:584-90. [Crossref] [PubMed]
- Sivaraj SM, Vimalraj V, Saravanaboopathy P, et al. Is bactibilia a predictor of poor outcome of pancreaticoduodenectomy? Hepatobiliary Pancreat Dis Int 2010;9:65-8. [PubMed]
- Ohgi K, Sugiura T, Yamamoto Y, et al. Bacterobilia may trigger the development and severity of pancreatic fistula after pancreatoduodenectomy. Surgery 2016;160:725-30. [Crossref] [PubMed]
- Kauhanen SP, Komar G, Seppänen MP, et al. A prospective diagnostic accuracy study of 18F-fluorodeoxyglucose positron emission tomography/computed tomography, multidetector row computed tomography, and magnetic resonance imaging in primary diagnosis and staging of pancreatic cancer. Ann Surg 2009;250:957-63. [Crossref] [PubMed]
- Asagi A, Ohta K, Nasu J, et al. Utility of contrast-enhanced FDG-PET/CT in the clinical management of pancreatic cancer: impact on diagnosis, staging, evaluation of treatment response, and detection of recurrence. Pancreas 2013;42:11-9. [Crossref] [PubMed]
- Crippa S, Salgarello M, Laiti S, et al. The role of (18)fluoro-deoxyglucose positron emission tomography/computed tomography in resectable pancreatic cancer. Dig Liver Dis 2014;46:744-9. [Crossref] [PubMed]
- Burge ME, O'Rourke N, Cavallucci D, et al. A prospective study of the impact of fluorodeoxyglucose positron emission tomography with concurrent non-contrast CT scanning on the management of operable pancreatic and peri-ampullary cancers. HPB (Oxford) 2015;17:624-31. [Crossref] [PubMed]
- Pimiento JM, Davis-Yadley AH, Kim RD, et al. Metabolic Activity by 18F-FDG-PET/CT Is Prognostic for Stage I and II Pancreatic Cancer. Clin Nucl Med 2016;41:177-81. [Crossref] [PubMed]
- Wang SL, Cao S, Sun YN, et al. Standardized uptake value on positron emission tomography/computed tomography predicts prognosis in patients with locally advanced pancreatic cancer. Abdom Imaging 2015;40:3117-21. [Crossref] [PubMed]
- Sakane M, Tatsumi M, Hori M, et al. Volumetric parameters of 2-deoxy-2-[18F]fluoro-d-glucose positron emission tomography/computed tomography can predict histopathologic treatment response after neoadjuvant chemoradiotherapy in pancreatic adenocarcinoma. Eur J Radiol 2017;94:64-9. [Crossref] [PubMed]
- Fiore M, Taralli S, Trecca P, et al. A Bio-Imaging Signature as a Predictor of Clinical Outcomes in Locally Advanced Pancreatic Cancer. Cancers (Basel) 2020;12:2016. [Crossref] [PubMed]
- Wartski M, Sauvanet A. 18F-FDG PET/CT in pancreatic adenocarcinoma: A role at initial imaging staging? Diagn Interv Imaging 2019;100:735-41. [Crossref] [PubMed]
- Patel M, Hoffe S, Malafa M, et al. Neoadjuvant GTX chemotherapy and IMRT-based chemoradiation for borderline resectable pancreatic cancer. J Surg Oncol 2011;104:155-61. [Crossref] [PubMed]
- Wang L, Dong P, Shen G, et al. 18F-Fluorodeoxyglucose Positron Emission Tomography Predicts Treatment Efficacy and Clinical Outcome for Patients With Pancreatic Carcinoma: A Meta-analysis. Pancreas 2019;48:996-1002. [Crossref] [PubMed]
- Sperti C, Friziero A, Serafini S, et al. Prognostic Implications of 18-FDG Positron Emission Tomography/Computed Tomography in Resectable Pancreatic Cancer. J Clin Med 2020;9:2169. [Crossref] [PubMed]
- Barnes CA, Aldakkak M, Clarke CN, et al. Value of Pretreatment (18)F-fluorodeoxyglucose Positron Emission Tomography in Patients With Localized Pancreatic Cancer Treated With Neoadjuvant Therapy. Front Oncol 2020;10:500. [Crossref] [PubMed]
- Zimmermann C, Distler M, Jentsch C, et al. Evaluation of response using FDG-PET/CT and diffusion weighted MRI after radiochemotherapy of pancreatic cancer: a non-randomized, monocentric phase II clinical trial-PaCa-DD-041 (Eudra-CT 2009-011968-11). Strahlenther Onkol 2021;197:19-26. [Crossref] [PubMed]
- Fiorentino A, Laudicella R, Ciurlia E, et al. Positron emission tomography with computed tomography imaging (PET/CT) for the radiotherapy planning definition of the biological target volume: PART 2. Crit Rev Oncol Hematol 2019;139:117-24. [Crossref] [PubMed]
- Gemenetzis G, Groot VP, Blair AB, et al. Incidence and risk factors for abdominal occult metastatic disease in patients with pancreatic adenocarcinoma. J Surg Oncol 2018;118:1277-84. [Crossref] [PubMed]
- Liu RC, Traverso LW. Diagnostic laparoscopy improves staging of pancreatic cancer deemed locally unresectable by computed tomography. Surg Endosc 2005;19:638-42. [Crossref] [PubMed]
- Karabicak I, Satoi S, Yanagimoto H, et al. Risk factors for latent distant organ metastasis detected by staging laparoscopy in patients with radiologically defined locally advanced pancreatic ductal adenocarcinoma. J Hepatobiliary Pancreat Sci 2016;23:750-5. [Crossref] [PubMed]
- Satoi S, Yanagimoto H, Yamamoto T, et al. A clinical role of staging laparoscopy in patients with radiographically defined locally advanced pancreatic ductal adenocarcinoma. World J Surg Oncol 2016;14:14. [Crossref] [PubMed]
- De Rosa A, Cameron IC, Gomez D. Indications for staging laparoscopy in pancreatic cancer. HPB (Oxford) 2016;18:13-20. [Crossref] [PubMed]
- Levy J, Tahiri M, Vanounou T, et al. Diagnostic Laparoscopy with Ultrasound Still Has a Role in the Staging of Pancreatic Cancer: A Systematic Review of the Literature. HPB Surg 2016;2016:8092109. [Crossref] [PubMed]
- Yamamura K, Yamashita YI, Yamao T, et al. Efficacy of Staging Laparoscopy for Pancreatic Cancer. Anticancer Res 2020;40:1023-7. [Crossref] [PubMed]
- Ta R, O'Connor DB, Sulistijo A, et al. The Role of Staging Laparoscopy in Resectable and Borderline Resectable Pancreatic Cancer: A Systematic Review and Meta-Analysis. Dig Surg 2019;36:251-60. [Crossref] [PubMed]



