
Haematological and nutritional prognostic biomarkers for patients receiving CROSS or FLOT
Highlight box
Key findings
• An elevated NLR at baseline is predictive of lower pathological response rates and poorer prognosis with worse survival outcomes for patients with upper gastrointestinal cancers treated with FLOT or CROSS regimens.
What is known and what is new?
• NLR has been shown to predict worse survival outcomes in multiple tumour types. Composite prognostic tools combine haematological and nutritional factors to predict outcomes.
• Lower rates of complete pathological response in those with an elevated NLR is a novel finding. Our study adds weight to the significant role inflammation plays in the tumour microenvironment.
What is the implication, and what should change now?
• Patients with a poorer prognosis may be identified by their baseline haematological and nutritional characteristics to inform clinical discussions and decisions. Further research is needed on the interplay of the host inflammatory state, cancer-specific inflammation and cancer cell growth.
Introduction
Globally, gastric cancer (GC) causes approximately 989,600 deaths a year, and oesophageal cancer (OC) causes 406,800 with both cancers disproportionately affecting men and those in developing countries (1). Multimodality treatment of locally advanced disease was established as the standard of care following publication of the carboplatin and paclitaxel concurrent chemoradiotherapy (CROSS) study, of neoadjuvant chemoradiation with carboplatin and paclitaxel, in 2012 (2). The FLOT4-AIO study of perioperative docetaxel, oxaliplatin, calcium folinate and fluorouracil (FLOT) has since been introduced as a treatment option for fit patients (3,4). Overall, outcomes remain reasonably poor for curative-intent treatment, with a trial population median of OS 48.6 months for the CROSS regimen and 50 months for fluorouracil (FLOT).
Clinically useful prognostic biomarkers are lacking for GCs and OCs beyond rudimentary staging with the American Joint Committee on Cancer (AJCC) TNM system (5). H. pylori, whilst a risk factor for initial and metachronous GCs, is not a marker of prognosis or treatment response (6).
Cancer-related inflammation and the intricacies of the tumour microenvironment continue to be explored in multiple tumour types. The role of tumour-promoting inflammation and the ability for cancer cells to evade immune destruction have now been held up as additional hallmarks of cancer by Hanahan and Weinberg (7). The response of the host immune system in the presence of malignancy can be readily, albeit coarsely, assessed by available haematological markers.
The neutrophil-lymphocyte ratio (NLR) and platelet-lymphocyte ratio (PLR) have been proposed as novel predictors of survival outcomes in metastatic GC, unresectable OC, and small cohorts of early OC (8,9). The utility of NLR as a predictor of response and survival was explored in patients treated with the now superseded regimen of ECF/X (epirubicin, cisplatin and fluorouracil or capecitabine) in 2020 (10). Powell et al. showed that a pre-treatment NLR of <2.25 was associated with improved pathological response rates and overall survival for oesophageal adenocarcinoma patients treated neoadjuvantly with cisplatin and fluorouracil (11). A PLR of ≥158 has been previously shown to be predictive or worse survival in a study of 199 patients by Jagadesham et al. in 2017 (12).
Similarly, readily available nutritional biomarkers of albumin and body mass index (BMI) have been explored as markers of cancer specific survival. Albumin has not been shown to have a statistically significant impact large enough to be detected in several studies (13,14). BMI has been shown to be itself predictive of survival outcomes in gastro-oesophageal junction (GOJ) cancers, GC and OC surgical patients, as well as a reduction in weight during treatment (14,15). Weight loss exceeding 2.75% per month was identified as an independent predictor of prognosis by Deans et al. in 2007 (14).
Our study aims to further evaluate baseline and dynamic NLR, PLR, albumin and BMI as predictors of survival outcomes, pathological response and toxicity in patients with OC, GC and GOJ cancers treated with neoadjuvant chemoradiation with the CROSS regimen and perioperative FLOT chemotherapy. We present the following article in accordance with the REMARK reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-886/rc).
Methods
This is a retrospective observational cohort study of patients with histologically confirmed locally advanced OC, GOJ or GC that were treated with at least one cycle of FLOT (5-fluorouracil 2,600 mg/m2 over 24 hours, leucovorin 50 mg, oxaliplatin 85 mg/m2 and docetaxel 50 mg/m2 given every 2 weeks for 8 cycles) or CROSS [carboplatin with area under the curve (AUC) of 2, paclitaxel 50 mg/m2 weekly with 1.8 Gy for 23 fractions] from January 2015 to June 2021 in 5 hospitals across Sydney, Australia. The seventh edition of the AJCC was used for patient staging based on endoscopic ultrasound, imaging and diagnostic laparoscopy. Management decisions were made in hospital-based multi-disciplinary team meetings for all patients. Toxicity data were graded and recorded using version 5 of the CTCAE (Common Terminology Criteria for Adverse Events). Patients identified to have metastatic disease prior to commencing therapy were excluded from the analysis.
DFS was measured from treatment initiation to the date of recurrence or progression or last follow-up. OS was measured from the date of completion of treatment to date of death or last known follow-up. NLR was calculated by the absolute neutrophil count divided by the absolute lymphocyte count and PLR was defined as absolute platelet count divided by the absolute lymphocyte count. Data were recorded for NLR, PLR, BMI and albumin at two time points for those treated with CROSS being prior to initiation of concurrent chemoradiotherapy and post-completion of chemoradiotherapy, prior to surgery. For patients treated with FLOT, the aforementioned variables were collected at three time points. These were prior to the first cycle of neoadjuvant FLOT, after the last cycle of neoadjuvant FLOT and after the last cycle of adjuvant FLOT. Pathological response at the time of surgery was also collected. Pathological response was assessed with either the modified Ryan scheme or AJCC-tumour regression grade (16).
Statistical analysis
Descriptive analysis was performed with Chi-square for categorical variables and t-test for continuous variables. The Kaplan-Meier method was used to graphically present survival curves. Hazard ratios for univariate and multivariate analysis were estimated with Cox-proportional regression. Multivariate analysis included baseline and sustained NLR, baseline and sustained PLR, baseline BMI, baseline albumin, pre- and post-treatment PET SUVmax for association with response, DFS or OS. A P value of 0.05 was considered statistically significant. All analysis was undertaken with R 4.1.1.
Ethical statement
This research was conducted with appropriate ethics approval through the Western Sydney Local Health District Human Research Ethics Committee. All research activities were performed in accordance with the Declaration of Helsinki (as revised in 2013). The need for informed consent was waived by the local Human Research Ethics Committee due to the retrospective nature of this study.
Results
Patient demographics
One hundred and sixty-eight patients were included. The median follow-up was 21.6 months with a median age of diagnosis of 66 (range, 25 to 83) years. In total 95 (57%) patients were treated with CROSS and 73 (43%) were treated with FLOT. The majority of patients were male (76%), with a performance status of Eastern Cooperative Oncology Group (ECOG) 0 (66.7%) and had adenocarcinoma (85%). Patient characteristics are presented in further detail in Table 1.
Table 1
| Variable | Total (N=168, %) | CROSS (N=95, %) | FLOT (N=73, %) |
|---|---|---|---|
| Age at diagnosis, years | |||
| <70 | 103 (61.3) | 49 (51.6) | 54 (74.0) |
| >70 | 65 (38.7) | 46 (48.4) | 19 (26.0) |
| Gender | |||
| Female | 41 (24.4) | 23 (24.2) | 18 (24.7) |
| Male | 127 (75.6) | 72 (75.8) | 55 (75.3) |
| ECOG | |||
| 0 | 98 (58.3) | 51 (53.7) | 47 (64.4) |
| 1+ | 49 (29.2) | 36 (37.9) | 13 (17.8) |
| Missing | 21 (12.5) | 8 (8.4) | 13 (17.8) |
| BMI, kg/m2 | |||
| <25 | 59 (38.4) | 31 (35.2) | 28 (38.4) |
| >25 | 102 (61.6) | 57 (64.8) | 45 (61.6) |
| Missing | 7 (4.2) | 7 (7.4) | 0 (0.0) |
| Tumour location | |||
| Gastric | 39 (23.2) | 0 (0.0) | 39 (53.4) |
| GOJ | 54 (32.1) | 32 (33.7) | 22 (30.1) |
| Oesophageal | 75 (44.6) | 63 (66.3) | 12 (16.4) |
| Histological subtype | |||
| Adenocarcinoma | 141 (84.9) | 68 (73.1) | 73 (100.0) |
| SCC | 25 (15.1) | 25 (26.9) | 0 (0) |
| Missing | 2 (1.2) | 2 (2.1) | 0 (0) |
| NLR | |||
| <2 | 48 (29.1) | 23 (25.0) | 25 (34.2) |
| ≥2 | 117 (70.9) | 69 (75.0) | 48 (65.8) |
| Missing | 3 (1.8) | 3 (3.2) | 0 (0) |
| PLR | |||
| <200 | 123 (73.7) | 71 (75.5) | 52 (71.2) |
| ≥200 | 44 (26.3) | 23 (24.5) | 21 (28.8) |
| Missing | 1 (0.6) | 1 (1.1) | 0 (0) |
| Pathological response | |||
| Complete | 30 (22.2) | 17 (23.0) | 13 (21.3) |
| Non-complete | 105 (77.8) | 57 (77.0) | 48 (78.7) |
| Missing | 33 (19.6) | 21 (22.1) | 12 (16.4) |
| Pathological stage | |||
| 0 | 30 (17.9) | 17 (17.9) | 13 (17.8) |
| I | 26 (15.5) | 24 (25.3) | 2 (2.7) |
| II | 38 (22.6) | 14 (14.7) | 24 (32.9) |
| III | 39 (23.2) | 19 (20.0) | 20 (27.4) |
| IV | 5 (3.0) | 2 (2.1) | 3 (4.1) |
| No surgery | 28 (16.7) | 17 (17.9) | 11 (15.1) |
| Missing | 2 (1.2) | 2 (2.1) | 0 (0.0) |
FLOT, fluorouracil, calcium folinate, oxaliplatin and docetaxel; CROSS, carboplatin and paclitaxel concurrent chemoradiotherapy; ECOG, Eastern Cooperative Oncology Group; BMI, body mass index; GOJ, gastro-oesophageal junction; SCC, squamous cell carcinoma; NLR, neutrophil-lymphocyte ratio; PLR, platelet-lymphocyte ratio.
The median follow-up for patients who received FLOT was 20.3 months. Patients who received FLOT had 53% GC, 30% GOJ and 16% OC. The median number of cycles received was 5 (range, 1 to 8) cycles. 63 (86%) patients received 4 cycles of FLOT but only 28 (38%) patients received all 8 cycles of FLOT and 12 (16%) did not require dose reductions throughout their treatment. 22 (30%) patients treated with FLOT had a relapse with a median DFS of 29.6 months.
The median follow-up for the CROSS cohort was 22.8 months. In total 32 (33.7%) patients had GOJ and 63 (66.3%) had OC. The majority of the CROSS cohort had adenocarcinoma (73.1%). In total 26 (27%) patients did not receive the full treatment, 22 (23%) had early cessation of chemotherapy whilst 4 (4%) patients did not receive the full 41.4 Gy in 23 fractions of radiotherapy. Thirty-nine (41%) patients who were treated with CROSS had a relapse and the median DFS was 28.5 months.
Neutrophil to lymphocyte ratio
An NLR of 2 was used to stratify patients after exploring ratios from 1.5 to 2.5 and finding 2 to be most significant. For our cohort on univariate analysis, a baseline NLR ≥2 was predictive for both OS (HR 2.90, 95% CI: 1.48–5.67, P<0.01) and DFS (HR 2.78, 95% CI: 1.41–5.50, P<0.01). Sustained elevation in NLR was also predictive for both DFS (HR 1.54, 95% CI: 1.08–2.17, P=0.01) and OS (HR 1.65, 95% CI: 1.17–2.33, P<0.01). This is demonstrated in the Kaplan-Meier survival curves in Figures 1,2. On multivariate analysis, summarised in Table 2, with additional stratification for treatment type in addition to the aforementioned variables, baseline NLR ≥2 was predictive of both worse OS (HR 3.48, 95% CI: 1.75–6.91, P<0.01) and DFS (HR 3.99, 95% CI: 1.10–14.4, P=0.03). Sustained elevation in NLR was also predictive for both DFS (HR 1.68, 95% CI: 1.18–2.39, P<0.01) and OS (HR 1.81, 95% CI: 1.28–2.58, P<0.01) on multivariate analysis. Treatment type between FLOT and CROSS did not influence survival outcomes.
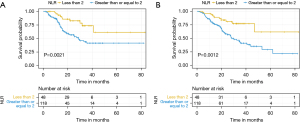
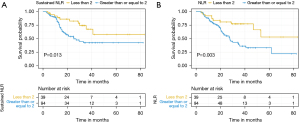
Table 2
| Variable | N (%) | HR for DFS in CROSS (95% CI, P value) |
HR for OS in CROSS (95% CI, P value) |
HR for DFS in FLOT (95% CI, P value) |
HR for OS in FLOT (95% CI, P value) |
HR for DFS combined (95% CI, P value) |
HR for OS combined (95% CI, P value) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline BMI, kg/m2 | |||||||||||||||
| <25 | 30 (34.5) | – | – | – | – | – | – | ||||||||
| >25 | 57 (65.5) | 1.07 (0.46–1.92, P=0.85) | 1.12 (0.47–1.72, P=0.74) | 1.75 (0.68–4.49, P=0.24) | 2.50 (0.92–6.77, P=0.07) | 1.04 (0.51–1.79, P=0.88) | 1.12 (0.64–1.96, P=0.68) | ||||||||
| Baseline NLR | |||||||||||||||
| <2 | 23 (24.7) | – | – | – | – | – | – | ||||||||
| ≥2 | 70 (75.2) | 3.80 (1.34–10.7, P=0.01) | 2.28 (1.02–5.13, P=0.04) | 1.99 (0.78–5.09, P=0.15) | 4.33 (1.28–14.6, P=0.01) | 3.99 (1.10–14.4, P=0.03) | 3.48 (1.74–6.91, P<0.01) | ||||||||
| Post-neoadjuvant NLR | |||||||||||||||
| <2 | 13 (14.6) | – | – | – | – | – | – | ||||||||
| ≥2 | 76 (85.3) | 1.26 (0.49–3.26, P=0.63) | 1.55 (0.61–3.96, P=0.36) | 1.63 (0.60–4.44, P=0.34) | 3.02 (0.88–10.3, P=0.07) | 1.55 (0.88–3.66, P=0.33) | 2.44 (0.71–9.33, P=0.21) | ||||||||
| Baseline PLR | |||||||||||||||
| <200 | 71 (76.3) | – | – | – | – | – | – | ||||||||
| ≥200 | 22 (23.7) | 1.41 (0.70–2.85, P=0.34) | 1.34 (0.70–2.57, P=0.37) | 0.97 (0.38–2.48, P=0.94) | 1.03 (0.38–2.48, P=0.94) | 1.32 (0.41–2.41, P=0.30) | 1.28 (0.55–2.67, P=0.41) | ||||||||
| Post-neoadjuvant PLR | |||||||||||||||
| <200 | 28 (30.1) | – | – | – | – | – | – | ||||||||
| ≥200 | 65 (69.9) | 0.98 (0.49–1.97, P=0.96) | 1.55 (0.78–3.07, P=0.21) | 1.49 (0.52–3.56, P=0.37) | 1.20 (0.48–2.97, P=0.69) | 1.23 (0.55–1.91, P=0.56) | 1.43 (0.65–2.11, P=0.31) | ||||||||
| Sustained high NLR | |||||||||||||||
| Not-sustained | 29 (32.6) | – | – | – | – | – | – | ||||||||
| Sustained | 60 (67.4) | 1.50 (1.01–2.25, P=0.03) | 1.40 (1.01–2.05, P=0.04) | 1.69 (0.80–3.56, P=0.17) | 1.45 (0.78–3.54, P=0.21) | 1.64 (0.96–2.12, P=0.12) | 1.45 (0.79–2.64, P=0.22) | ||||||||
| Sustained high PLR | |||||||||||||||
| Not-sustained | 68 (80.9) | – | – | – | – | – | – | ||||||||
| Sustained | 16 (19.0) | 1.13 (0.76–1.69, P=0.54) | 1.15 (0.79–1.66, P=0.46) | 1.36 (0.27–1.16, P=0.25) | 1.05 (0.50–1.80, P=0.86) | 1.11 (0.48–2.11, P=0.71) | 1.23 (0.53–1.98, P=0.62) | ||||||||
| Albumin | |||||||||||||||
| ≥33 | 11 (11.8) | – | – | – | – | – | – | ||||||||
| <33 | 82 (88.2) | 6.61 (0.90–48.44, P=0.06) | 3.82 (0.92–15.88, P=0.06) | 2.35 (0.55–10.0, P=0.25) | 2.85 (0.66–12.2, P=0.16) | 6.17 (1.47–25.9, P=0.01) | 4.66 (1.42–15.2, P=0.01) | ||||||||
HR, hazard ratio; DFS, disease free survival; OS, overall survival; CROSS, carboplatin and paclitaxel concurrent chemoradiotherapy; FLOT, fluorouracil, calcium folinate, oxaliplatin and docetaxel; BMI, body mass index; NLR, neutrophil-lymphocyte ratio; PLR, platelet-lymphocyte ratio.
Baseline NLR, when assessed as a continuous variable, was also shown to be predictive of pathological complete response (pCR) with those patients achieving a pCR having a mean NLR of 2.1 compared with an NLR of 3.1 in those who did not have pCR (P<0.01). When patients were dichotomised by NLR, 48% (15/31) of patients with baseline NLR <2 had a pCR compared with 15% (15/98) in those with baseline NLR ≥2 which was a statistically significant finding (Chi-square 4.01, P=0.04).
Platelet to lymphocyte ratio
The median baseline PLR was 136 for CROSS patients and 145 for FLOT patients. Baseline PLR was not predictive for either DFS or OS when assessed as continuous variable nor when stratified at cut-offs of 150, 175 or 200. A high post-chemoradiotherapy PLR or sustained PLR was not associated with either DFS or OS for patients treated with CROSS. Similarly, post treatment PLR or sustained PLR was not associated with either DFS or OS for patients treated with FLOT. Baseline or dynamic PLR was not associated with toxicity outcomes.
Nutritional and FDG-PET markers
On multivariate analysis, a baseline albumin <33 was predictive of worse DFS with a HR of 6.17 (P=0.01) as well as worse OS with a HR of 4.66 (P=0.01). There was no correlation between baseline albumin and rates of pCR when assessed as a continuous variable or when stratified at using 32 as the cut-off. BMI and albumin were not associated with toxicity outcomes. The dynamic BMI data was not able to be assessed due to inconsistent documentation in clinical record systems. Neither pre-treatment SUV nor post-treatment SUV were predictive of pCR rates or survival outcomes.
Discussion
The data presented here have expanded on the work of other centres. We have demonstrated that readily available haematological and nutritional markers can be used to inform survival outcomes for patients receiving FLOT or CROSS regimens. Our key findings are that both an elevated baseline and sustained NLR as well as baseline hypoalbuminaemia are predictive of worse survival for these patients. This is a significant finding in the context of a wider conversation in multiple tumour groups about how we understand the role of the immune system and inflammatory state. The poor prognosis that an elevated NLR yields is consistent with the available literature that was reviewed prior to commencing this project.
The finding of a statistically significant difference in the rates of pCR between NLR high and low patients is consistent with the associated DFS and OS findings. This finding in our study adds to the growing body of evidence for improved response rates in those with a low NLR. Having a pCR after neoadjuvant therapy has previously been established to directly correlate with improved survival, as is the case in other tumour types (17). The NLR is a coarse reflection of the host inflammatory state which could be easily assessed on peripheral blood count. Neutrophil-derived inflammatory proteins have been shown to be involved in tumour cell invasion and migration (18,19). Consistent with our results, a low NLR has been shown to correlate with improved outcomes in a number of malignancies including breast cancer, hepatocellular carcinoma and upper gastrointestinal cancers (20-22).
The PLR results were not suggestive of any predictive value in our study. In addition to the previously mentioned study by Jagadesham et al. which used a PLR of 158, a larger study of 492 patients examining whether PLR predicted the presence of lymph node metastases in GC used a PLR cut-off of 155.67 (12,23). This may suggest that either our study may be too small to detect this signal or that our PLR value was too high. However even analysis using alternative PLR values, there was no correlation with outcomes. Another explanation may be that the role of the PLR is a less relevant reflection of the host immune response compared to the NLR.
Current studies examining the immunogenicity of chemotherapy may further inform our choice of chemotherapy regimen. Whilst there was a small increase in the proportion of patients with an elevated NLR after neoadjvuant systemic therapy, our study was not large enough to draw meaningful conclusions from this. Davern et al. have demonstrated in vitro that FLOT and CROSS regimens upregulated the expression of immune checkpoint receptors in oesophageal adenocarcinoma leading to increased immune resistance (24). This opens the future direction of combining immune checkpoint inhibition with neoadjuvant and adjuvant regimens for these patients. The phase IIb DANTE trial has reported interim results with improved response rates when atezolizumab is added to perioperative FLOT for gastric or gastroesophageal adenocarcinomas (25).
Our finding of a serum albumin <33 correlating with worse survival is a significant finding when considering the lack of existing data in this area. This patient group of CROSS and FLOT recipients has limited nutritional survival data currently available in the literature. A previous study of 199 patients who received neoadjuvant chemotherapy for GOJ cancer concluded that sarcopaenia develops during treatment but did not impact outcomes (26). Similarly a descriptive study of 69 patients undergoing treatment for GOJ and OC cancer had a median weight loss of 10.5%, but no conclusions could be drawn on its relationship to outcomes (27). Our data clearly show worse outcomes for those with a low albumin.
The lack of significant findings involving BMI, on either baseline or dynamic analyses, suggests that this may be an imperfect marker of nutrition, or indeed that nutrition itself plays only a small role in predicting survival. Additional higher quality evidence is needed to elucidate the role of nutrition for this group of patients. The prospective PREHAB study established in France to answer this question planned to recruit 120 patients to be randomised to pre-operative nutritional and exercise support of standard of care treatment, however results are yet to be published (NCT02780921) (28).
Regarding toxicity, our study found no correlation between these haematological and nutritional markers and toxicity outcomes. Whilst there is a paucity of high-quality studies examining these questions, our findings were consistent with available evidence (29). Sarcopaenia is prevalent in upper gastrointestinal cancer patients and worsens with therapy, however hasn’t been shown to be associated with treatment related toxicity (30).
To increase the power of our study, our analysis combined the FLOT and CROSS cohorts. Importantly in the multivariate analysis, reported results were independent of the treatment received suggesting homogeneity from this dataset. This is in keeping with other studies which have explored the inflammatory state in this cohort of patients (14,31). A further limitation of our multivariate analysis is the unavailability of pre-treatment lymph node status and surgical margin status, factors which are known to have significant prognostic value.
Further exploration of the prognostic and predictive value of the NLR and how that can assist patient and clinician decision making should be generated out of this project. A more sophisticated analysis of the nature of the NLR and the specific role it plays in tumour proliferation and angiogenesis may yield prognostic tools beyond the simple ratios presented here.
Conclusions
We have demonstrated that a high baseline inflammatory state represented by an elevated baseline NLR of ≥2 is both prognostic and predictive of response in patients undergoing treatment with CROSS or FLOT regimens. Hypoalbuminaemia was also predictive of worse survival. These results could aid prognostication discussions and lead to further studies of the interaction between treatment and host immune response in GC, GOJ and OCs. Further analyses with large cohorts are needed to confirm these results and explore their impact on clinical decision making.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-886/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-886/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-886/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-886/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This research was conducted with appropriate ethics approval through the Western Sydney Local Health District Human Research Ethics Committee. All research activities were performed in accordance with the Declaration of Helsinki (as revised in 2013). The need for informed consent was waived by the local Human Research Ethics Committee due to the retrospective nature of this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Al-Batran SE, Homann N, Pauligk C, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet 2019;393:1948-57. [Crossref] [PubMed]
- Owens R, Cox C, Gomberg S, et al. Outcome of Weekly Carboplatin-Paclitaxel-based Definitive Chemoradiation in Oesophageal Cancer in Patients Not Considered to be Suitable for Platinum-Fluoropyrimidine-based Treatment: A Multicentre, Retrospective Review. Clin Oncol (R Coll Radiol) 2020;32:121-30. [Crossref] [PubMed]
- Rice TW, Ishwaran H, Blackstone EH, et al. Recommendations for clinical staging (cTNM) of cancer of the esophagus and esophagogastric junction for the 8th edition AJCC/UICC staging manuals. Dis Esophagus 2016;29:913-9.
- Choi IJ, Kook MC, Kim YI, et al. Helicobacter pylori Therapy for the Prevention of Metachronous Gastric Cancer. N Engl J Med 2018;378:1085-95. [Crossref] [PubMed]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [Crossref] [PubMed]
- Li KJ, Xia XF, Su M, et al. Predictive value of lymphocyte-to-monocyte ratio (LMR) and neutrophil-to-lymphocyte ratio (NLR) in patients with oesophageal cancer undergoing concurrent chemoradiotherapy. BMC Cancer 2019;19:1004. [Crossref] [PubMed]
- Wang H, Ding Y, Li N, et al. Prognostic Value of Neutrophil-Lymphocyte Ratio, Platelet-Lymphocyte Ratio, and Combined Neutrophil-Lymphocyte Ratio and Platelet-Lymphocyte Ratio in Stage IV Advanced Gastric Cancer. Front Oncol 2020;10:841. [Crossref] [PubMed]
- Bradley CA. Perioperative FLOT superior to ECF/X. Nat Rev Clin Oncol 2019;16:465. [Crossref] [PubMed]
- Powell AGMT, Chin C, Coxon AH, et al. Neutrophil to lymphocyte ratio as a predictor of response to neoadjuvant chemotherapy and survival in oesophageal adenocarcinoma. BJS Open 2020;4:416-23. [Crossref] [PubMed]
- Jagadesham VP, Lagarde SM, Immanuel A, et al. Systemic inflammatory markers and outcome in patients with locally advanced adenocarcinoma of the oesophagus and gastro-oesophageal junction. Br J Surg 2017;104:401-7. [Crossref] [PubMed]
- Crumley AB, McMillan DC, McKernan M, et al. An elevated C-reactive protein concentration, prior to surgery, predicts poor cancer-specific survival in patients undergoing resection for gastro-oesophageal cancer. Br J Cancer 2006;94:1568-71. [Crossref] [PubMed]
- Deans DA, Wigmore SJ, de Beaux AC, et al. Clinical prognostic scoring system to aid decision-making in gastro-oesophageal cancer. Br J Surg 2007;94:1501-8. [Crossref] [PubMed]
- Tamandl D, Paireder M, Asari R, et al. Markers of sarcopenia quantified by computed tomography predict adverse long-term outcome in patients with resected oesophageal or gastro-oesophageal junction cancer. Eur Radiol 2016;26:1359-67. [Crossref] [PubMed]
- Ryan R, Gibbons D, Hyland JM, et al. Pathological response following long-course neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Histopathology 2005;47:141-6. [Crossref] [PubMed]
- Homann N, Pauligk C, Luley K, et al. Pathological complete remission in patients with oesophagogastric cancer receiving preoperative 5-fluorouracil, oxaliplatin and docetaxel. Int J Cancer 2012;130:1706-13. [Crossref] [PubMed]
- Shamamian P, Schwartz JD, Pocock BJ, et al. Activation of progelatinase A (MMP-2) by neutrophil elastase, cathepsin G, and proteinase-3: a role for inflammatory cells in tumor invasion and angiogenesis. J Cell Physiol 2001;189:197-206. [Crossref] [PubMed]
- Huh SJ, Liang S, Sharma A, et al. Transiently entrapped circulating tumor cells interact with neutrophils to facilitate lung metastasis development. Cancer Res 2010;70:6071-82. [Crossref] [PubMed]
- Matsuura K, Osaki A, Nakame A, Fujimoto A, Ichinose Y, Nukui A, et al. MO32-7 High ALC, low NLR and PLR are associated with longer OS in patients with metastatic breast cancer treated with eribulin. Annals of oncology. 2021;32:S319-S.
- Zhou L, Wang J, Lyu SC, et al. PD-L1(+)NEUT, Foxp3(+)Treg, and NLR as New Prognostic Marker with Low Survival Benefits Value in Hepatocellular Carcinoma. Technol Cancer Res Treat 2021;20:15330338211045820. [Crossref] [PubMed]
- Booka E, Kikuchi H, Haneda R, et al. Neutrophil-to-Lymphocyte Ratio to Predict the Efficacy of Immune Checkpoint Inhibitor in Upper Gastrointestinal Cancer. Anticancer Res 2022;42:2977-87. [Crossref] [PubMed]
- Pang W, Lou N, Jin C, et al. Combination of preoperative platelet/lymphocyte and neutrophil/lymphocyte rates and tumor-related factors to predict lymph node metastasis in patients with gastric cancer. Eur J Gastroenterol Hepatol 2016;28:493-502. [Crossref] [PubMed]
- Davern M, Donlon NE, Sheppard A, et al. Chemotherapy regimens induce inhibitory immune checkpoint protein expression on stem-like and senescent-like oesophageal adenocarcinoma cells. Transl Oncol 2021;14:101062. [Crossref] [PubMed]
- Al-Batran SE, Lorenzen S, Thuss-Patience PC, et al. Surgical and pathological outcome, and pathological regression, in patients receiving perioperative atezolizumab in combination with FLOT chemotherapy versus FLOT alone for resectable esophagogastric adenocarcinoma: Interim results from DANTE, a randomized, multicenter, phase IIb trial of the FLOT-AIO German Gastric Cancer Group and Swiss SAKK. J Clin Oncol 2022;40:4003. [Crossref]
- den Boer RB, Jones KI, Ash S, et al. Impact on postoperative complications of changes in skeletal muscle mass during neoadjuvant chemotherapy for gastro-oesophageal cancer. BJS Open 2020;4:847-54. [Crossref] [PubMed]
- Mak M, Bell K, Ng W, et al. Nutritional status, management and clinical outcomes in patients with esophageal and gastro-oesophageal cancers: A descriptive study. Nutr Diet 2017;74:229-35. [Crossref] [PubMed]
- Le Roy B, Pereira B, Bouteloup C, et al. Effect of prehabilitation in gastro-oesophageal adenocarcinoma: study protocol of a multicentric, randomised, control trial-the PREHAB study. BMJ Open 2016;6:e012876. [Crossref] [PubMed]
- Rinninella E, Strippoli A, Cintoni M, et al. Body Composition Changes in Gastric Cancer Patients during Preoperative FLOT Therapy: Preliminary Results of an Italian Cohort Study. Nutrients 2021;13:960. [Crossref] [PubMed]
- Elliott JA, Doyle SL, Murphy CF, et al. Sarcopenia: Prevalence, and Impact on Operative and Oncologic Outcomes in the Multimodal Management of Locally Advanced Esophageal Cancer. Ann Surg 2017;266:822-30. [Crossref] [PubMed]
- Wagener-Ryczek S, Schoemmel M, Kraemer M, et al. Immune profile and immunosurveillance in treatment-naive and neoadjuvantly treated esophageal adenocarcinoma. Cancer Immunol Immunother 2020;69:523-33. [Crossref] [PubMed]


