
Compared to preoperative plasma levels post-operative urokinase-type plasminogen activator-1 levels are persistently elevated for 6 weeks after minimally invasive colorectal resection
Highlight box
Key findings
• uPA plasma levels were shown to be significantly elevated for over one month after MICR in CRC patients.
What is known and what is new?
• Preop blood levels of uPA were noted to be elevated in a wide range of cancers including colorectal tumors. The current study, the first to assess uPA levels postoperatively, shows, for reasons not likely related to cancer, that after bowel resection plasma uPA levels are significantly increased over preop baseline for about 1 month.
What is the implication, and what should change now?
• uPA and the other long duration elevated angiogenic proteins after surgery are capable of promoting tumor development via mechanisms related to angiogenesis. Further investigation to develop anti-cancer treatments to be used perioperatively that are effective but that do not interfere with wound healing appears warranted.
Introduction
In the United States, colorectal cancer (CRC) is the third leading cause of cancer-related deaths, with 13% 5-year survival in patients with metastasis (1). Surgical resection remains the mainstay of treatment although advances in chemotherapy and radiotherapy have increased the 5-year survival rate notably. Adjuvant chemotherapy, when indicated, is usually delayed for 4 to 6 weeks after surgery to allow for wound healing. Of note, there is anecdotal clinical evidence and strong experimental evidence suggesting that the growth of residual tumor deposits is stimulated in the early postoperative (postop) period (2-6). Although the origin of these tumor-promoting modifications is uncertain, some investigators have implicated surgery-related immunosuppression and/or persistent proangiogenic and tumor stimulatory alterations in plasma protein levels that continue for 2–5 weeks following surgery (7). Thus far, long duration plasma increases have been noted for a total of 14 proangiogenic proteins that include angiopoietin-2 (Ang-2), vascular endothelial growth factor (VEGF), placental growth factor (PLGF), and multiple matrix metalloproteinases (MMPs) (4-11). Also, plasma from weeks 2 and 3 following minimally invasive surgery (MIS) with removal of CRC has been shown to promote endothelial cell (EC) migration and invasion in vitro (12). These changes in proangiogenic compositions within the plasma may in fact foster promotion of tumor angiogenesis within remaining tumor deposits early after surgery. To better define surgery’s impact on blood composition, investigators continue to assess blood levels of proteins known to play a role in tumor growth and spread. Urokinase-type plasminogen activator-1 (uPA) is one such protein that could, by virtue of its many potential effects, impact tumor cell behavior if significantly elevated levels in the plasma were present.
During and soon after wounding, fibrin plugs play an important hemostatic role. However, during the wound healing proliferative phase, fibrin is degraded by plasmin and other enzymes and is replaced with collagen. uPA and tissue plasminogen activator (t-PA), which convert inactive plasminogen to plasmin, play key roles in this process. In addition to its role in fibrin degradation, plasminogen/plasmin and their associated regulatory proteins, including uPA and its receptor, urokinase-type plasminogen activator receptor (uPAR), are capable of breaking down the basement membrane and extracellular matrix (ECM). The uPA-uPAR system also activates MMP, growth factors and other proteases and also interacts with integrin and vitronectin. In the setting of cancer, collectively, these effects break down the physical barrier to tumor cell migration and facilitate tumor invasion and metastasis as well as stimulate tumor growth. These effects of uPA/uPAR also support tumor angiogenesis which is critical to tumor growth. This study of CRC patients concerns uPA which a key component of the plasmin activation system. If uPA levels are persistently increased postop, similar to the above-mentioned proteins with long duration plasma elevations, then further evidence will have been found to support the hypothesis that colorectal resection is associated plasma protein changes that are stimulatory to tumor growth. The level of uPA protein and/or mRNA has been noted to be elevated in a significant proportion of breast, ovarian, bladder, gastric, prostate, and CRCs (13-23). Further, elevated tumor uPA levels have been associated with a worse outcome or more advanced disease for all of these cancers (13-18,21-23). Also, plasma levels of uPA have been shown to be significantly elevated in patients with ovarian, endometrial, non-small cell lung cancer (NSCLC), gastric, hepatocellular, pancreatic, colorectal, and bladder cancers (24-28).
Although preoperative blood levels of uPA have been studied, the influence of surgical resections on levels of postop plasma has not been investigated. The purpose of this study is to determine levels of plasma uPA before, as well as during the first 6 weeks following minimally invasive colorectal resection (MICR) in a group of CRC patients. We present the following article in accordance with the MDAR reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-113/rc).
Methods
Study population
Adult CRC patients at Mount Sinai West Hospital, New York and New York Presbyterian Hospital, Columbia University, New York who had undergone elective MICR and had consented preoperatively to enrollment for an Institutional Review Board (IRB)-approved data and tissue banking protocol for subjects that had sufficient quantities of pre and postop plasma available were eligible for this study. The preoperative plasma levels, determined prior to incision, provide the baseline uPA levels against which the postop results are compared.
Ideally, this study would also have included CRC patients who underwent open surgical resection to allow for an assessment of the contribution of surgical method on the results noted, however, the volume of open resections in our centers was insufficient to allow inclusion of an open surgery arm.
The time period across which these patients underwent surgery was 2007 to 2016. One purpose of this banking effort was to determine the impact of major surgery on blood protein composition. Demographic, clinical, peri-operative, and pathologic data was collected for all consenting patients. Immunosuppressed patients and those that had undergone chemotherapy or radiotherapy within 4 weeks of surgery were excluded. Patients who received blood transfusions perioperatively were also not eligible.
Blood samples and enzyme-linked immunosorbent assay (ELISA)
Blood samples were obtained from all patients preoperatively and on postop days (PODs) 1, 3, and at least 1 timepoint beyond the first week postop. It was not possible to obtain late blood specimens on set postop days after discharge from the hospital for logistical reasons, therefore, the specimens received late were grouped into 7-day time blocks (POD 7–13, 14–20, and 21–27) which were in turn considered individual points of time for data analysis. Of note, because of the small number of samples obtained during postop weeks 5 and 6, the blood samples from those 2 weeks were grouped together and viewed as a single time point to permit statistical analysis.
Blood samples were obtained in heparin containing tubes (Cat No. 367878Becton Dickinson, New Jersey, USA) and the plasma isolated via centrifugation within 6 hours and stored in 500 µL aliquots at −80 ℃ until utilized.
An ELISA that was available commercially for uPA was utilized for the assays in accordance with manufacturer instructions (Cat No. DUPA00, R&D Systems, Minneapolis, USA). Plasma uPA levels were determined in duplicate and 8 serial dilution standard curve samples were included on each 96 well plate; the results are reported as ng/mL. Briefly, 50 µL three times diluted (with calibrator diluent supplied with the kit) plasma samples in duplicate were used. Diluted human uPA stock solution (20,000 pg/mL) provided with the kit was used to make the standard curve. The standard curve was made using the 2,000 pg/mL uPA as the high standard and calibrator diluent as the zero standards (0 pg/mL). The optical density of ELISA plate was determined at 450 nm at the end of the reaction using an automated microplate reader (Synergy2; Bio-Tek Instruments, Inc., Winooski, VT, USA). The mean plasma uPA concentrations of samples were determined using the log/log curve-fit standard curve.
Statistical analysis
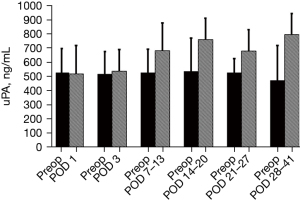
Continuous random variables such as age, surgical time, length of stay (LOS), surgical incision size of group were presented as average and SD whereas for categorical variables, frequencies and percentages were utilized. Because the blood samples obtained after discharge were not obtained on set PODs, and because the specimens received late were spread out over a period of 3 to 4 weeks, the later samples were then grouped into 7-day time blocks (POD 7–13, 14–20, 21–27, and 28–41) and were interpreted as single time points for the sake of data analysis. Because values of uPA for pre-operative and corresponding postop time points were not distributed normally for time points collected later, comparisons of uPA levels for the preop vs. Postop time points were performed with the use of non-parametric (Wilcoxon signed rank paired) test, after which the data were reported utilizing median along with confidence interval (CI) values. Also, a bar graph was used to depict the data expressing levels of uPA as median with a 75% quartile range (Figure 1). We believe this graph is more suitable to explain the difference of preop vs. postop uPA level at each time points. Comparisons of hand assisted procedure subgroup preop and post op values vs. laparoscopy assisted procedure subgroup preop and post op values, advancing cancer stage subgroup values, male vs. female subgroups values were analyzed by nonparametric Mann and Whitney test since comparisons were done between different groups and numbers (n) of each group were small. Correlation between postop plasma uPA levels and age as well as length of surgery was analyzed by Spearman’s rank correlation coefficient (rs). A P value of P<0.05 was used as statistically significant. All data analysis was performed using SPSS version 15.0 (SPSS, Inc., Chicago, IL, USA). Manuscript is updated accordingly.
Ethical statement
The study was conducted by using material collected from patients who consented preoperatively to participate in the Mount Sinai West Colorectal service’s IRB-approved general tissue and data banking protocol (No. GCO1: 16-2619-Institutional Review Board of the Mount Sinai School of Medicine, New York; and IRB reference No. AAAA4473-IRB of the Columbia University Medical Center, New York). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from all participating CRC patients who were enrolled in an IRB approved data/plasma bank and all patients assented to analysis, present and to publish the paper.
Results
A total of 93 eligible CRC patients who underwent MICR were selected for the study. There were 46 males and 47 females with a mean age of 65.6±13.9 years. Sixty-three (68%) patients had colon cancer whereas 30 (32%) had rectal malignancies. The breakdown of the types of resections carried out is shown is Table 1; operations performed most commonly were: right hemicolectomy (29.0%), low anterior or anterior resection (20.4%), sigmoidal resection (21.5%), and abdominoperineal resection (7.5%). As regards surgical method, a laparoscopic-assisted operation was done in 61% [mean incision length (IL) 6.96±3.9 cm] and a hand-assisted laparoscopic operation was done in 39% of patients (mean incision size, 10.0±2.7 cm). There were 10 conversions in the laparoscopy assisted group (defined as incision greater than 7 cm) and 6 conversions in the hand-assisted MIS group (incision greater than 12 cm). The operative time mean value was 336.5±117.7 minutes. The LOS mean value was 7.9±3.7 days. When 4 outliers are eliminated (LOS 35, 16, 15, 15 days) the mean LOS is 6.2 days. The following complications were noted: ileus [9], urinary retention [8], urinary tract infection [3], cardiac [3], pneumonia [2], pulmonary edema [2], sepsis [2], phlebitis [2], wound infection [1], Clostridium difficile colitis [1], seroma [1], and seroma [1]. There were no leaks or intrabdominal abscesses. There were no reoperations, however, 1 patient had a percutaneous gastrostomy tube. There were no deaths within 30 days. The final cancer stage breakdown follows; stage I (30%), stage II (30%), stage III (34%) and stage IV (6%).
Table 1
| Variables | Values |
|---|---|
| Age (years), mean ± SD | 65.6±13.3 |
| Sex, n (%) | |
| Male | 46 (49.5) |
| Female | 47 (50.5) |
| Incision size (total study population) (cm), mean ± SD | 7.0±4.1 |
| Incision size (laparoscopic procedure group) (cm), mean ± SD | 6.96±3.9 |
| Incision size (hand-assisted procedure group) (cm), mean ± SD | 9.96±3.2 |
| Operative time (min), mean ± SD | 336.5±117.7 |
| Length of stay (days), mean ± SD | 7.9±3.7 |
| Type of resection, n (%) | |
| Right | 27 (29.0) |
| Sigmoid/recto-sigmoid [14/6] | 20 (21.5) |
| LAR/AR [14/5] | 19 (20.4) |
| Proctectomy/total/sub-total [5/3/1] | 9 (9.7) |
| APR | 7 (7.5) |
| Transverse | 6 (6.5) |
| Left | 5 (5.4) |
| Surgical method, n (%) | |
| Laparoscopic procedure-assisted | 59 (63.4) |
| Hand-assisted/hybrid laparoscopic (HAL) | 34 (36.6) |
| Conversion, lap-assisted to HAL or open (final incision greater than 7 cm) | 10 (10.8) |
| Conversion, HAL to open (incision >12 cm) | 6 (6.5) |
| Colostomies | 9 (9.7) |
| Ileostomies (diverting + permanent) | 11 (11.8) |
APR, abdominoperineal resection; AR, anterior resection; LAR, low anterior resection; SD, standard deviation.
When compared to median preop plasma levels of uPA (529.8, 95% CI: 462.8, 601.1), significant elevations in median levels (ng/mL) were noted POD 3 (542.8, 95% CI: 518.8, 597.3, n=86, P=0.003), POD 7–13 (688.1, 95% CI: 591.7, 753.0, n=72, P<0.001), POD 14–20 (764.9, 95% CI: 704.1, 911.6, n=27, P<0.001), POD 21–27 (685.6, 95% CI: 443.8, 835.8, n=15, P<0.001) and on POD 28–41 (800.3, 95% CI: 626.9, 940.6, n=21, P<0.001) (Figure 1). The increased percentage from mean baseline at each individual time point was: 8.3% (POD 1), 10.6% (POD 3), 43.6% (POD 7–13), 66.3% (POD 14–20), 30.8% (POD 21–27), and 40.5% (POD 28–41).
Of note, a significant correlation was not found between age and pre-operative or postop uPA levels. Similarly, there were no significance differences noted in the uPA levels between the male and female patients pre- or postoperatively. Of note, there was no association demonstrated concerning cancer stage and preop uPA levels. Also, no significant differences were found in uPA levels between the hand-assisted laparoscopic group (mean IL 9.96±3.2 cm) and the lap-assisted subgroup (mean IL 6.96±3.9 cm) at any of the postop time points. Of note, the colon cancer subgroup’s preop and POD 14–20 mean results were significantly higher than the corresponding rectal cancer results; non-significant elevations were also noted in the colon subgroup on POD 7–13, 21–27, and 28–41. There was no relationship found between length of surgery and postop plasma uPA levels.
Discussion
The vast majority of serum protein changes that occur in response to surgery and anesthesia resolve within days (29,30); examples include interleukin (IL)-1 receptor antagonist, TNF, IL-6, and C-reactive protein (CRP) (31). These transient alterations are thought to be related to the acute inflammatory and the hypothalamic-pituitary-adrenal axis responses to tissue trauma and anesthesia. In contrast to these short-lived plasma compositional changes, this study of CRC patients who underwent MICR, found that the mean serum level of uPA was significantly elevated for almost a month after surgery. The peak elevations were noted during the second and third weeks after surgery.
Although similar plasma uPA changes were noted in the colon and rectal cancer subgroups for most time points, the colon group had significantly higher levels preop and at 1 postop time point. The age and sex of the patient as well as the specific method used in surgery utilized (lap-assisted vs. hand-assisted MIS) was not significant in impacting the postop results. What is the cause of the uPA plasma increases after surgery?
This study did not assess or consider possible etiologies, therefore, we can only speculate as to the cause(s) of these changes. Whereas, the acute inflammatory response might contribute to the early increase it cannot account for the week 2 to 4 elevations. Since the cancer has been removed it is highly unlikely that the malignancy is the cause. Of note, there is human evidence regarding etiology for 8 proteins for which plasma levels were similarly increased following MICR for 3 to 5 weeks (32). The study in question simultaneously assessed blood and wound fluid levels of 8 proteins at multiple time points for up to 3 weeks following MICR for both CRC and benign colon pathology. Wound fluid was obtained from the abdomen/pelvis or subcutaneous wounds via Jackson Pratt drains placed at surgery. The median postop plasma levels of the 8 proteins were, again, found to be significantly and persistently elevated from their preop baselines. The wound fluid levels of the same proteins were noted as 3 to 40 times above the correspondingly higher plasma levels at all time points. These results raised the possibility that the increased levels of proteins in the blood originated in healing wounds and had diffused into the blood stream. The fact that all 8 proteins have been shown to play some role in wound healing lends support to this hypothesis. These results also suggest that wound healing is a lengthy process that influences plasma composition. This hypothesis can also be invoked to explain uPA’s persistent elevation since uPA’s principal action is the activation of plasmin which plays a critical role in the healing of wounds. Plasmin degrades fibrin clots in wounds, and provides access to fibroblasts, ECs, and other cells involved in tissue repair and angiogenesis. What is known about uPA?
Pro-uPA is released by a large variety of cell types including ECs, fibroblasts, neutrophils, monocytes, and macrophages as a single polypeptide chain (411 amino acids) with minimal plasmin activating function. It is cleaved into a 2 chain “activated” high molecular weight (HMW) form by plasmin, kallikrein, cathepsin, trypsin and other enzymes. uPA can be further degraded into a single chain low molecular weight (LMW) form that maintains plasmin activating activity. Pro-uPA, HMW uPA, as well as LMW uPA can all bind to cell surface bound uPAR. uPA is broken down and regulated by the serine proteases PAI-1 and PAI-2. uPA and the uPA-uPAR complex, besides generating plasmin, activates MMP and other growth factors [basic fibroblast growth factor (bFGF), transforming growth factor β (TGF-β), IL-1β]; uPA-uPAR also impacts cell adhesion by interacting with vitronectin. uPA-uPAR is not capable of independent signal transduction, however, because it has multiple binding sites it can interact with integrins and initiate intracellular signaling via the Jak-STAT, focal adhesion kinase (FAK), and other pathways that influence transcription, cell growth and survival (33,34). These effects and actions facilitate tissue repair and wound healing. As noted, plasmin and plasmin activator system also figure prominently in cancer development.
Plasmin is the principal mechanism by which ECM and the basement membrane are degraded in solid tumors. ECM breakdown provides tumor cells access to adjacent tissues, lymphatics, and blood vessels which facilitates invasion, metastases, and angiogenesis. uPA, by activating plasmin plays a crucial role in this process (33). Also, uPA, independent of plasmin, and in conjunction with its cell bound receptor uPAR, facilitates tumor growth via multiple pathways (35,36). uPA alone has been shown to positively modulate extracellular regulated kinase’s (ERK’s) tumor stimulatory effects in breast cancer cells; in addition, when bound to uPAR, it enhances the latter’s independent ERK/p38 pro-tumor growth modulating activity (37,38). As regards angiogenesis, it has also been demonstrated that uPA has chemotactic effects on endothelial and smooth muscle cells and is a mediator of the mitogenic effects of PDGF and bFGF on vascular smooth muscle cells (39,40). uPA alone and when bound to uPAR has also been shown to activate and promote several growth factors and to modulate both cell proliferation and apoptosis (34). Also of note, the uPA activation and degradation process generates an amino terminal fragment that has been shown to have mitogenic effects and to stimulate EC movement (41-43).
As mentioned in the introduction, uPA expression is notably increased in many types of tumors and high expression has been shown to be a negative prognostic indicator. Also, preop blood levels were noted to be elevated in a wide range of cancers including colorectal tumors. The current study, the first to assess uPA levels postoperatively, shows, for reasons not likely related to cancer, that after bowel resection plasma uPA levels are significantly increased over preop baseline for about 1 month. Thus, uPA joins the short but growing list of proteins that have been demonstrated to remain elevated up to and beyond one month postoperatively. This list includes angiopoietin-2, chemokine ligand 16, IL-8, chitinase-3 like protein, MMP-3, monocyte chemotactic protein-1, PLGF, PAI-1, soluble vascular adhesion molecule-1, and finally VEGF (4-6,8-12,29).
VEGF plays crucial role in promoting multiple steps early in angiogenesis. VEGF triggers proliferation of ECs along with, migration and invasion thus encouraging continued development of microvasculature, whereas Ang-2 promotes destabilization of connections between endothelium and perivascular cells, which enrich the effects of VEGFs (8). In addition to the proliferation and survival of ECs, PLGF stimulates recruitment of the smooth muscle precursors enveloping developing vessels in tumors. Taken together along with VEGF, these factors produce more stable and mature vessels (10). Soluble vascular cell adhesion molecule-1 (sVCAM-1) supports a vital beginning step in the development of angiogenesis via binding to VLA4 in vitro which is EC-bound and induces chemotaxis of ECs via both FAK as well as p38 mitogen-activated protein (MAP) signaling pathways (9,44,45). Monocyte chemoattractant protein-1 (MCP-1), via the recruitment of proangiogenic proteins, promotes angiogenesis by generating both plasma monocytes as well as tissue macrophages and ECs into wounds and tumors (5). In tumor stroma, macrophages have shown in previous studies to express CHi3L1, which likely stimulates tumor angiogenesis (6,46). Protease family protein MMPs 2, 3, and 7 are key regulators of cell matrix interactions and support angiogenesis by way of promoting stimulation of budding vessels as well as migrating cells (47-49). CXCL-16, a transmembrane chemokine, and its receptor CXCR-6 pair activity associated with pro-inflammatory stimuli, signaling induced recruitment and angiogenesis of endothelial precursor cells (4,50-52). IL-8 offers a function in angiogenesis, often by healing surgical wounds tissues (11) and uPA, by activating plasmin plays a crucial role in ECM breakdown, invasion, metastases, and angiogenesis. Studies utilizing ECs housed in vitro suggest the end effect of plasma make-up changes during postop weeks 2 through 3 is proangiogenic. uPA is also part of the group of proteins that exude blood levels after MICR. It is apparent from the biological role of these proteins including uPA that they all have some effect in the process of angiogenesis.
As mentioned, all of these proteins have some effect in the process of angiogenesis. Further, human plasma from the postop weeks 2 through 3 after colorectal resection has been previously shown to stimulate proliferation, invasion, and migration of ECs in vitro (8,12). Thus, the postop plasma is proangiogenic for up to a month. Also of note, uPA and the other long duration elevated proteins after surgery are capable of promoting tumor development via mechanisms related to angiogenesis. Are these changes sufficient and capable of stimulating tumor angiogenesis and growth in residual metastases? If so, then the immediate postop period may be a dangerous time in the life of cancer patients because adjuvant chemotherapy is routinely not started for 4 to 6 weeks. In that case, it would make sense to develop anti-cancer treatments to be used perioperatively that are effective but that do not interfere with wound healing. Immunomodulators and other immunotherapies are options that warrant assessment for this time period. It is the authors view that further study of surgery’s systemic impact on the patient’s physiology is indicated. Also, investigations focused on elucidating the downstream manifestations and effects of wound healing would also be of value.
This study has a number of shortcomings. First the late postop time point sample sizes are notably less than the overall sample size. Unfortunately, it is not possible to obtain weekly samples from all patients after discharge for logistical reasons. Patients are geographically scattered and have, at most 2 office visits during the first 4 to 6 weeks at which time samples can be obtained. A second limitation is that the late samples are bundled into 7-day time periods. This is a necessity because it is not possible to schedule postop office visits such that they fall on the same postop day. A third weakness is that we did not assess baseline uPA levels in a control group of cancer free patients and thus cannot comment on whether the cancer patients preop levels are elevated preoperatively. Finally, the study patients’ tumors were not analyzed for uPA expression. Finally, we have no intermediate or long term oncologic follow up data.
Conclusions
uPA plasma levels were shown to be elevated for over one month after MICR in CRC patients. The size of the increase peaked during the second postop week and the percent change from baseline for postop weeks 2 through 6 ranged from 30 to 66 percent. Of unclear importance and for unclear reasons the uPA levels of the subgroup of colon cancer patients were higher than the subgroup of rectal cancer patients at 2/7 time points. Of note, age, sex, and the use of laparoscopic assisted vs. hand assisted methods did not impact postop levels. The pathogenesis of the postop change in uPA is not unknown and was not addressed in this study. However, based on a prior study done on a group of proteins that demonstrated similar persistent increases in blood levels after CRC surgery, it is postulated that the healing wounds may be one cause. Because all 15 of the proteins shown to elevated for 3–6 weeks after MICR promote angiogenesis and/or tumor growth, further investigation of this time period and the advancement of anti-cancer therapies for early postop use appears warranted.
Acknowledgments
The authors acknowledge the Wade Thomson Foundation for generous donation of funds to the Division of Colon and Rectal Surgery, Department of Surgery, Mount Sinai West Hospital, New York to complete this study.
The abstract has been presented in American Association of Cancer Research meeting 2022 April 8-13, Louisiana USA, as a poster and published in Cancer Res [2020;80(16_Supplement):3937].
Funding: This study was supported by a generous donation from the Thompson Family Foundation to the Division of Colon and Rectal Surgery, Department of Surgery, Mount Sinai West Hospital, New York.
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-113/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-113/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-113/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The present study was conducted by using material collected from patients who consented preoperatively to participate in the Mount Sinai West Colorectal service’s IRB-approved general tissue and data banking protocol (No. GCO1: 16-2619-Institutional Review Board of the Mount Sinai School of Medicine, New York; and IRB reference No. AAAA4473-Institutional Review Board of the Columbia University Medical Center, New York presbyterian hospital). Written informed consent was obtained from all participating colorectal cancer patients who were enrolled in an IRB approved data/plasma bank and all patients assented to analysis, present and to publish the paper.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Chung CC, Ng DC, Tsang WW, et al. Hand-assisted laparoscopic versus open right colectomy: a randomized controlled trial. Ann Surg 2007;246:728-33. [Crossref] [PubMed]
- Coffey JC, Wang JH, Smith MJ, et al. Excisional surgery for cancer cure: therapy at a cost. Lancet Oncol 2003;4:760-8. [Crossref] [PubMed]
- Shantha Kumara HMC, Pettke E, Shah A, et al. Plasma levels of the proangiogenic protein CXCL16 remains elevated for 1 month after minimally invasive colorectal cancer resection. World J Surg Oncol 2018;16:132. [Crossref] [PubMed]
- Shantha Kumara HM, Myers EA, Herath SA, et al. Plasma monocyte chemotactic protein-1 remains elevated after minimally invasive colorectal cancer resection. World J Gastrointest Oncol 2014;6:413-9. [Crossref] [PubMed]
- Shantha Kumara HM, Gaita D, Miyagaki H, et al. Plasma chitinase 3-like 1 is persistently elevated during first month after minimally invasive colorectal cancer resection. World J Gastrointest Oncol 2016;8:607-14. [Crossref] [PubMed]
- Tang F, Tie Y, Tu C, et al. Surgical trauma-induced immunosuppression in cancer: Recent advances and the potential therapies. Clin Transl Med 2020;10:199-223. [Crossref] [PubMed]
- Kumara HM, Feingold D, Kalady M, et al. Colorectal resection is associated with persistent proangiogenic plasma protein changes: postoperative plasma stimulates in vitro endothelial cell growth, migration, and invasion. Ann Surg 2009;249:973-7. Erratum in: Ann Surg 2009 Dec;250(6):1046. [Crossref] [PubMed]
- Shantha Kumara HM, Tohme ST, Herath SA, et al. Plasma soluble vascular adhesion molecule-1 levels are persistently elevated during the first month after colorectal cancer resection. Surg Endosc 2012;26:1759-64. [Crossref] [PubMed]
- Shantha Kumara HM, Cabot JC, Yan X, et al. Minimally invasive colon resection is associated with a persistent increase in plasma PlGF levels following cancer resection. Surg Endosc 2011;25:2153-8. [Crossref] [PubMed]
- Shantha Kumara HMC, Sutton E, Bellini GA, et al. Plasma interleukin-8 levels are persistently elevated for 1 month after minimally invasive colorectal resection for colorectal cancer. Mol Clin Oncol 2018;8:471-6. [Crossref] [PubMed]
- Shantha Kumara HM, Kirchoff D, Naffouje S, et al. Plasma from the second and third weeks after open colorectal resection for cancer stimulates in vitro endothelial cell growth, migration, and invasion. Surg Endosc 2012;26:790-5. [Crossref] [PubMed]
- Fisher JL, Field CL, Zhou H, et al. Urokinase plasminogen activator system gene expression is increased in human breast carcinoma and its bone metastases--a comparison of normal breast tissue, non-invasive and invasive carcinoma and osseous metastases. Breast Cancer Res Treat 2000;61:1-12. [Crossref] [PubMed]
- Li S, Wei X, He J, et al. Plasminogen activator inhibitor-1 in cancer research. Biomed Pharmacother 2018;105:83-94. [Crossref] [PubMed]
- Grossmann NC, Schuettfort VM, Pradere B, et al. Further Understanding of Urokinase Plasminogen Activator Overexpression in Urothelial Bladder Cancer Progression, Clinical Outcomes and Potential Therapeutic Targets. Onco Targets Ther 2021;14:315-24. [Crossref] [PubMed]
- Kimura S, D'Andrea D, Iwata T, et al. Expression of urokinase-type plasminogen activator system in non-metastatic prostate cancer. World J Urol 2020;38:2501-11. [Crossref] [PubMed]
- Kita Y, Fukagawa T, Mimori K, et al. Expression of uPAR mRNA in peripheral blood is a favourite marker for metastasis in gastric cancer cases. Br J Cancer 2009;100:153-9. [Crossref] [PubMed]
- Märkl B, Hardt J, Franz S, et al. Tumor Budding, uPA, and PAI-1 in Colorectal Cancer: Update of a Prospective Study. Gastroenterol Res Pract 2017;2017:6504960. [Crossref] [PubMed]
- Choong PF, Nadesapillai AP. Urokinase plasminogen activator system: a multifunctional role in tumor progression and metastasis. Clin Orthop Relat Res 2003;S46-58. [Crossref] [PubMed]
- Kaneko T, Konno H, Baba M, et al. Urokinase-type plasminogen activator expression correlates with tumor angiogenesis and poor outcome in gastric cancer. Cancer Sci 2003;94:43-9. [Crossref] [PubMed]
- Tang L, Han X. The urokinase plasminogen activator system in breast cancer invasion and metastasis. Biomed Pharmacother 2013;67:179-82. [Crossref] [PubMed]
- Costantini V, Sidoni A, Deveglia R, et al. Combined overexpression of urokinase, urokinase receptor, and plasminogen activator inhibitor-1 is associated with breast cancer progression: an immunohistochemical comparison of normal, benign, and malignant breast tissues. Cancer 1996;77:1079-88. [Crossref] [PubMed]
- Ding Y, Zhang H, Zhong M, et al. Clinical significance of the uPA system in gastric cancer with peritoneal metastasis. Eur J Med Res 2013;18:28. [Crossref] [PubMed]
- Nantajit D, Chailapakul P, Bawornpatarapakorn S, et al. Prognostic significance of uPA and uPAR expression in patients with cervical cancer undergoing radiotherapy. Oncol Lett 2021;21:423. [Crossref] [PubMed]
- Herszényi L, István G, Cardin R, et al. Serum cathepsin B and plasma urokinase-type plasminogen activator levels in gastrointestinal tract cancers. Eur J Cancer Prev 2008;17:438-45. [Crossref] [PubMed]
- Yang SF, Hsieh YS, Lin CL, et al. Increased plasma levels of urokinase plasminogen activator and matrix metalloproteinase-9 in nonsmall cell lung cancer patients. Clin Chim Acta 2005;354:91-9. [Crossref] [PubMed]
- Itoh T, Hayashi Y, Kanamaru T, et al. Clinical significance of urokinase-type plasminogen activator activity in hepatocellular carcinoma. J Gastroenterol Hepatol 2000;15:422-30. [Crossref] [PubMed]
- Shariat SF, Monoski MA, Andrews B, et al. Association of plasma urokinase-type plasminogen activator and its receptor with clinical outcome in patients undergoing radical cystectomy for transitional cell carcinoma of the bladder. Urology 2003;61:1053-8. [Crossref] [PubMed]
- Shantha Kumara HM, Tohme ST, Kim IY, et al. Minimally invasive colorectal resection is associated with a transient increase in plasma hepatocyte growth factor levels early after surgery for colon cancer. Surg Innov 2011;18:254-8. [Crossref] [PubMed]
- Ordemann J, Jacobi CA, Schwenk W, et al. Cellular and humoral inflammatory response after laparoscopic and conventional colorectal resections. Surg Endosc 2001;15:600-8. [Crossref] [PubMed]
- Jawa RS, Anillo S, Huntoon K, et al. Interleukin-6 in surgery, trauma, and critical care part II: clinical implications. J Intensive Care Med 2011;26:73-87. [Crossref] [PubMed]
- Shantha Kumara H, Yan XH, Pettke E, et al. Plasma and wound fluid levels of eight proangiogenic proteins are elevated after colorectal resection. World J Gastrointest Oncol 2019;11:470-88. [Crossref] [PubMed]
- Mahmood N, Mihalcioiu C, Rabbani SA. Multifaceted Role of the Urokinase-Type Plasminogen Activator (uPA) and Its Receptor (uPAR): Diagnostic, Prognostic, and Therapeutic Applications. Front Oncol 2018;8:24. [Crossref] [PubMed]
- Hildenbrand R, Gandhari M, Stroebel P, et al. The urokinase-system--role of cell proliferation and apoptosis. Histol Histopathol 2008;23:227-36. [Crossref] [PubMed]
- Mars WM, Zarnegar R, Michalopoulos GK. Activation of hepatocyte growth factor by the plasminogen activators uPA and tPA. Am J Pathol 1993;143:949-58.
- Irigoyen JP, Muñoz-Cánoves P, Montero L, et al. The plasminogen activator system: biology and regulation. Cell Mol Life Sci 1999;56:104-32. [Crossref] [PubMed]
- Ma Z, Webb DJ, Jo M, et al. Endogenously produced urokinase-type plasminogen activator is a major determinant of the basal level of activated ERK/MAP kinase and prevents apoptosis in MDA-MB-231 breast cancer cells. J Cell Sci 2001;114:3387-96. [Crossref] [PubMed]
- Aguirre-Ghiso JA, Estrada Y, Liu D, et al. ERK(MAPK) activity as a determinant of tumor growth and dormancy; regulation by p38(SAPK). Cancer Res 2003;63:1684-95.
- Padró T, Mesters RM, Dankbar B, et al. The catalytic domain of endogenous urokinase-type plasminogen activator is required for the mitogenic activity of platelet-derived and basic fibroblast growth factors in human vascular smooth muscle cells. J Cell Sci 2002;115:1961-71. [Crossref] [PubMed]
- Rabbani SA, Desjardins J, Bell AW, et al. An amino-terminal fragment of urokinase isolated from a prostate cancer cell line (PC-3) is mitogenic for osteoblast-like cells. Biochem Biophys Res Commun 1990;173:1058-64. [Crossref] [PubMed]
- Tkachuk V, Stepanova V, Little PJ, et al. Regulation and role of urokinase plasminogen activator in vascular remodelling. Clin Exp Pharmacol Physiol 1996;23:759-65. [Crossref] [PubMed]
- Rabbani SA, Mazar AP, Bernier SM, et al. Structural requirements for the growth factor activity of the amino-terminal domain of urokinase. J Biol Chem 1992;267:14151-6.
- Odekon LE, Sato Y, Rifkin DB. Urokinase-type plasminogen activator mediates basic fibroblast growth factor-induced bovine endothelial cell migration independent of its proteolytic activity. J Cell Physiol 1992;150:258-63. [Crossref] [PubMed]
- Nakao S, Kuwano T, Ishibashi T, et al. Synergistic effect of TNF-alpha in soluble VCAM-1-induced angiogenesis through alpha 4 integrins. J Immunol 2003;170:5704-11. [Crossref] [PubMed]
- Koch AE, Halloran MM, Haskell CJ, et al. Angiogenesis mediated by soluble forms of E-selectin and vascular cell adhesion molecule-1. Nature 1995;376:517-9. [Crossref] [PubMed]
- Libreros S, Garcia-Areas R, Iragavarapu-Charyulu V. CHI3L1 plays a role in cancer through enhanced production of pro-inflammatory/pro-tumorigenic and angiogenic factors. Immunol Res 2013;57:99-105. [Crossref] [PubMed]
- Shantha Kumara HM, Gaita DJ, Miyagaki H, et al. Minimally invasive colorectal resection is associated with significantly elevated levels of plasma matrix metalloproteinase 3 (MMP-3) during the first month after surgery which may promote the growth of residual metastases. Surg Endosc 2014;28:3322-8. [Crossref] [PubMed]
- Lee EJ, Kim SY, Hyun JW, et al. Glycitein inhibits glioma cell invasion through down-regulation of MMP-3 and MMP-9 gene expression. Chem Biol Interact 2010;185:18-24. [Crossref] [PubMed]
- Shantha Kumara H, Miyagaki H, Herath SA, et al. Plasma MMP-2 and MMP-7 levels are elevated first month after surgery and may promote growth of residual metastases. World J Gastrointest Oncol 2021;13:879-92. [Crossref] [PubMed]
- Yoshie O, Imai T, Nomiyama H. Chemokines in immunity. Adv Immunol 2001;78:57-110. [Crossref] [PubMed]
- Isozaki T, Arbab AS, Haas CS, et al. Evidence that CXCL16 is a potent mediator of angiogenesis and is involved in endothelial progenitor cell chemotaxis: studies in mice with K/BxN serum-induced arthritis. Arthritis Rheum 2013;65:1736-46. [Crossref] [PubMed]
- Kim CH, Kunkel EJ, Boisvert J, et al. Bonzo/CXCR6 expression defines type 1-polarized T-cell subsets with extralymphoid tissue homing potential. J Clin Invest 2001;107:595-601. [Crossref] [PubMed]


