
Gelatin sponge microparticles for transarterial chemoembolization combined with regorafenib in hepatocellular carcinoma: a single-center retrospective study
Highlight box
Key findings
• GSMs-TACE combined with regorafenib may be effective and safe in the treatment of advanced HCC.
What is known and what is new?
• Conventional iodine TACE (c-TACE) combined regorafenib is active in advanced HCC. However, the liver damage of c-TACE limits its application in large advanced HCC;
• GSMs-TACE combined with regorafenib has shown positive effects in the treatment of advanced HCC. The efficacy may be related to the degree of embolization and the initial dose of regorafenib.
What is the implication, and what should change now?
• Patients with advanced HCC who have failed first-line sorafenib or lenvatinib may benefit from combination therapy. However, the conclusion of this study still needs to be confirmed by large-scale prospective cohort studies.
Introduction
Hepatocellular carcinoma (HCC) is a highly aggressive malignancy that accounts for 75–80% of all primary liver cancers (1,2). Transarterial chemoembolization (TACE) is widely used in HCC, and has been accepted as the recommended treatment for HCC by the European Association for the Study of the Liver (EASL), the American Society of Clinical Oncology (ASCO), and other guidelines (3-6). As a local treatment, TACE is recommended for HCC at Barcelona Clinic Liver Cancer (BCLC) stage 0, BCLC stage A, and partial BCLC stage B. TACE is also considered to be an important treatment for unresectable HCC in which systemic antitumor therapy is recommended (6-8). To date, relevant clinical studies have shown positive results in the treatment of advanced progressive HCC with TACE combined with systemic therapy such as multi-kinase inhibitors and/or immunotherapy (9-12).
Tyrosine kinase inhibitors (TKIs) are one of the common systemic treatment drugs for liver cancer. Sorafenib, lenvatinib, and regorafenib are common TKI drugs used in HCC (6). Their antitumor effects are achieved primarily by blocking and suppressing the activity of various protein kinases involved in tumor angiogenesis, tumorigenesis, metastasis, and tumor immunity (13-17). Regorafenib is the first drug to show a significant survival advantage in second-line treatment after the progression of first-line sorafenib or lenvatinib therapy in HCC patients (5,17). Some studies have shown that TACE combined with sorafenib or lenvatinib can provide better survival benefits than TACE alone or drug therapy alone (9,18). Moreover, some studies involving a combination of TACE and regorafenib in the treatment of unresectable HCC have also shown positive results (19).
Currently, a single-center retrospective study has shown that conventional transarterial chemoembolization (c-TACE) combined with regorafenib has a positive effect on unresectable HCC (19). In which, the median progression-free survival (PFS) and median overall survival (OS) were 9.1 and 14.3 months respectively, and the maximum tumor size was 3.75 (0.9, 15.1) cm. However, advanced HCC is often characterized by large tumor size, multiple intrahepatic tumors and portal vein tumor thrombosis (PVTT), while c-TACE is highly likely to lead to liver failure in the treatment of large HCC, which limits the further application of c-TACE in advanced liver cancer. Therefore, a combination of TACE technology with minimal liver damage and regorafenib needs to be further explored Clinically. Gelatin sponge microparticles for hepatic transarterial chemoembolization (GSMs-TACE) is a TACE technique involving the use of absorbable gelatin sponge particles. Our previous studies have shown efficacy and safety of GSMs-TACE in the treatment of primary liver cancer and liver metastasis of colorectal and breast cancer (20-22). particularly, previous study in our center have shown that GSMs-TACE has a positive effect in the treatment of BCLC stage C and large HCC (23). while, there is no literature reporting the efficacy and safety of GSMs-TACE combined with regorafenib. Therefore, this retrospective study was conducted to initially explore the efficacy and safety of GSMs-TACE combined with regorafenib in patients with unresectable advanced HCC who failed to responds to first-line sorafenib and/or lenvatinib therapy. We present the following article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1170/rc).
Methods
Patients
This single-center retrospective study reviewed the case data of patients who progressed after sorafenib and/or lenvatinib from December 2018 to June 2021 at the Affiliated Zhongshan Hospital of Dalian University in China. The diagnosis for HCC was based on imaging or biopsy analyses (24). The study was approved by ethics committee of Affiliated Zhongshan Hospital of Dalian University (No. 2021022-1) and was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Individual consent for this retrospective analysis was waived. The inclusion criteria were as follows: (I) 25 to 80 years old; (II) pathologically or clinically diagnosed HCC; (III) unresectable or refusing resection; (IV) progression after sorafenib and/or lenvatinib treatment; (V) BCLC stage B or C; (VI) Child-Pugh grade A or B; (VII) ECOG-PS score of 0–2; (VIII) Life expectancy >2 months; and (Ⅸ) received GSMs-TACE combined with regorafenib. The exclusion criteria were as follows: (I) incomplete baseline and follow-up information; (II) severe cardiovascular and cerebrovascular diseases, severe kidney injury and blood diseases were detected; (III) with other malignancies or other malignancies cured in less than 6 years.
The last follow-up date was June 2021. The sample size was determined by the number of patients who satisfied the inclusion and exclusion criteria during the study period.
Treatment
The GSMs-TACE was performed by experienced interventionalists with more than 10 years of clinical practice to ensure quality. The femoral artery approach was used in all cases. Depending on the tumor blood supply, gelatin sponge particles with the size of 350–560 or 560–710 µm were mixed with 10 mg lobaplatin and used for embolization. The complete or incomplete embolization of tumor vessels depended on the patient’s liver function, degree of cirrhosis, and tumor size. Within 1 week after GSMs-TACE, patients commenced oral regorafenib at different doses. Patients could choose to maintain or reduce the dose of regorafenib depending on the severity of adverse events (AEs), if any.
Data collection and definitions
All data were collated from the electronic health records. The clinical baseline data such as pathologic diagnosis rate, BCLC stage, α-fetal protein (AFP), ECOG score, Child-Pugh grade, macrovascular invasion, extrahepatic spread, cirrhosis, hepatitis B/C infection, maximum lesion diameter, number of intrahepatic lesions, complications, and previous treatment were collated. The sessions and frequency of TACE, reason for termination of TACE, initial dose of regorafenib, course of treatment, duration, dosage adjustment, and reason for discontinuation were defined as treatment-related variables. Enhanced computed tomography (CT) and magnetic resonance imaging (MRI) scans were used to determine tumor location, size, and tumor enhancement areas.
Follow-up was conducted every 3 to 5 weeks after combination therapy. If the treatment was changed due to disease progression, the patients were followed up every 3 months to obtain OS time. Follow-up ended upon death from any cause. The primary outcomes were OS and PFS. The secondary outcomes were complete response (CR), partially resolved (PR), stable disease (SD), progressive disease (PD), objective response rate (ORR), and disease control rate (DCR) [according to modified Response Evaluation Criteria In Solid Tumors (mRECIST)]. Clinical efficacy of treatment was assessed by one experienced imaging physicians and one interventional physician to reduce the measurement bias. Drug safety was evaluated according to the Common Terminology Criteria for Adverse Events (CTCAE 5.03). In this study, high and low AFP levels were defined as AFP <400 and ≥400 ng/mL, respectively, and the low or high initial regorafenib referred to ≤80 and ≥120 mg/d, respectively.
Statistical analysis
R.4.2.0 (R foundation for Statistical computing, Vienna, Austria) and Excel (Windows Excel 2019, Microsoft, Redmond, WA, USA) software were used for statistical analyses. Kolmogorov-Smirnov test is used to evaluate the normal distribution characteristics of continuous variables. Continuous variables with normally distribution data are expressed as mean ± standard deviation, and with skewed are shown as median (interquartile range). Categorical variables are represented as n (%). Kaplan-Meier method and log-rank test were used for survival analysis. Prognostic factors affecting PFS and OS were analyzed by univariate and multivariate Cox regression analyses. We first used univariate Cox regression analysis. When P<0.05 in univariate analysis, variables will be further included in multivariate analysis. Two-sided P values <0.05 were considered statistically significant.
Results
Basic patient characteristics
A total of 54 patients satisfied the inclusion criteria. After excluding 4 patients with missing baseline data, 2 patients with severe renal insufficiency, and 1 patient with previous breast cancer, a total of 47 patients were included in the study. All patients had received one or more local treatments before enrollment, including 44 patients who received TACE (93.6%), 8 patients with ablation (17.0%), and 7 surgical patients (14.9%). A total of 24 patients (51.1%) received sorafenib, 15 patients (31.9%) received lenvatinib, and 8 patients (17.0%) received lenvatinib after sorafenib. The age of patients was 64.4±6.8 years, and the median follow-up time of the patients was 11.6 months [95% confidence interval (CI): 10.8 to 13.9 months]. The detailed clinical baseline data of the patients are shown in Table 1.
Table 1
| Classification | Total (n=47) |
|---|---|
| Age (years), mean ± SD | 64.4±6.8 |
| Gender, n (%) | |
| Male | 43 (91.5) |
| Female | 4 (8.5) |
| ECOG-PS score, n (%) | |
| 1 or less | 23 (48.9) |
| 2 | 24 (51.1) |
| Child-Pugh grade, n (%) | |
| A | 22 (46.8) |
| B | 25 (53.2) |
| Tumor size (cm), median (IQR) | 5.1 (3.8, 8.9) |
| Number of intrahepatic tumors, n (%) | |
| <4 | 15 (31.9) |
| ≥4 | 32 (68.1) |
| AFP, n (%) | |
| <400 ng/mL | 20 (42.6) |
| ≥400 ng/mL | 27 (57.4) |
| Type of hepatitis, n (%) | |
| HBV | 34 (72.3) |
| HCV | 2 (4.3) |
| None | 11 (23.4) |
| BCLC grade, n (%) | |
| B | 14 (29.8) |
| C | 33 (70.2) |
| Portal vein invasion, n (%) | 21 (44.7) |
| Extrahepatic metastasis, n (%) | 24 (51.1) |
| Pathological diagnosis, n (%) | 38 (80.9) |
SD, standard deviation; ECOG, Eastern Cooperative Oncology Group; PS, performance status; IQR, interquartile range; AFP, α-fetoprotein; HBV, hepatitis B virus; HCV, hepatitis C virus; BCLC, Barcelona Clinic Liver Cancer.
Treatment profile of GSMs-TACE combined with regorafenib
The median number of GSMs-TACE sessions was 3. Different sizes of gelatin sponge microparticles mixed with lobaplatin were used to embolize the tumor artery, including 21 (44.7%) patients who used 350–560 µm microparticles, 6 (12.8%) patients who used 560–710 µm particles, and 20 (42.6%) patients who used a combination of the 2 sizes. The number of patients with the initial dose of regorafenib 80, 120 and 160 mg/d were 17 (36.2%), 23 (48.9%) and 7 (14.9%), respectively. Six (12.8%) patients experienced AEs of grade 3/4, and 36 (76.6%) patients presented with AEs of grade 1/2 during treatment. Two patients discontinued treatment due to AEs, and 11 (23.4%) patients reduced the dose due to side effects.
Outcomes
At the last follow-up, 17 (36.2%) patients were alive, 2 (4.3%) were lost to follow-up, and 28 (59.6%) patients had died. The median follow-up time was 11.6 months (95% CI: 10.8 to 14.0 months). The median progression-free survival (mPFS) was 6.0 months (95% CI: 4.5 to 7.5 months) and the median overall survival (mOS) was 14.3 months (95% CI: 11.8 to 16.8 months). The cumulative survival rates at 3, 6, 12, 18, and 24 months were 95.7%, 91.4%, 62.3%, 31.5%, and 15.1%, respectively (Figure 1). In terms of response rate, 2 (4.3%) patients experienced CR, PR was observed in 8 (17.0%) patients, SD in 26 (55.3%) patients, and PD in 11 (23.4%) patients. The ORR and DCR were 21.3% and 85.1%, respectively (Table 2). Patients achieving CR, PR, or SD had a longer PFS (log-rank P<0.001) and OS (log-rank P<0.001) than those with PD (Figure 2).
Table 2
| Best response | Total (n=47), n (%) |
|---|---|
| Complete response | 2 (4.3) |
| Partially resolved | 8 (17.0) |
| Stable disease | 26 (55.3) |
| Disease progression | 11 (23.4) |
| Objective response rate | 10 (21.3) |
| Disease control rate | 36 (76.6) |

Univariable and multivariable analyses
In this study, univariate analysis of Cox was used as the variable outcome of PFS. Patients classified as Child-Pugh A were associated with longer PFS than patients classified as Child-Pugh B [log-rank P=0.015, hazard ratio (HR) =0.485, 95% CI: 0.261 to 0.902, P=0.022]. Patients with AFP levels ≥400 ng/mL were associated with longer PFS than patients with AFP levels <400 ng/mL (log-rank P=0.047, HR =0.557, 95% CI: 0.303 to 1.023, P=0.059). BCLC B was associated with longer PFS than BCLC C (log-rank P=0.005, HR =0.408, 95% CI: 0.208 to 0.799, P=0.009). Patients without PVTT were associated with longer PFS than those with PVTT (log-rank P=0.013, HR =0.460, 95% CI: 0.240 to 0.883, P=0.020). Patients with complete embolization at TACE were associated with longer PFS than those with incomplete embolization (log-rank P<0.001, HR =3.383, 95% CI: 1.760 to 6.504, P<0.001). Patients with an initial dose of 120 or 160 mg/d regorafenib were associated with longer PFS than patients on 80 or 40 mg/d regorafenib (log-rank P=0.001, HR =2.642, 95% CI: 1.427 to 4.892, P=0.002). After adjusting for Child-Pugh grading, AFP levels, BCLC stage, and PVTT in the Cox multivariable model, complete embolization (HR =2.271, 95% CI: 1.023 to 5.041, P=0.044) and initial dosage of 120 or 160 mg/d regorafenib (HR =2.233, 95% CI: 1.102 to 4.526, P=0.026) were found to be independently associated with PFS (Figure 3A).
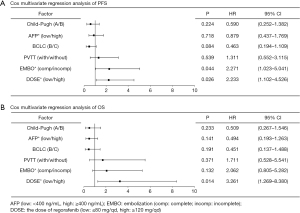
Cox univariable analysis was then performed with OS as the outcome variable. Child-Pugh A patients were associated with longer OS than Child-Pugh B patients (log-rank P=0.019, HR =0.410, 95% CI: 0.188 to 0.895, P=0.025). AFP levels <400 ng/mL were associated with longer OS than AFP levels ≥400 ng/mL (log-rank P=0.018, HR =0.386, 95% CI: 0.171 to 0.873, P=0.022). BCLC B was associated with longer OS than BCLC C (log-rank P=0.011, HR =0.339, 95% CI: 0.140 to 0.820, P=0.016). Patients without PVTT were associated with longer OS than those with PVTT (log-rank P=0.030, HR =0.435, 95% CI: 0.199 to 0.948, P=0.036). Complete embolization at TACE was associated with longer OS than incomplete embolization (log-rank P=0.004, HR =2.953, 95% CI: 1.367 to 6.378, P=0.006). An initial dose of 120 or 160 mg/d regorafenib was associated with longer OS compared to an initial dose of 80 or 40 mg/d (log-rank P=0.001, HR =3.359, 95% CI: 1.576 to 8.193, P=0.002). After adjusting for Child-Pugh grading, AFP levels, BCLC stage, PVTT, and embolization degree in the Cox multivariable model, Cox multivariable regression analysis revealed that OS was related to the initial dose of regorafenib (HR =3.261, 95% CI: 1.269 to 8.380, P=0.014; Figure 3B).
Adverse events
As shown in Table 3, complications after TACE included nausea (n=21, 44.7%), pain (n=28, 59.6%), vomiting (n=8, 17.0%), and fever (n=10, 21.3%). No grade 5 AEs (deaths) were observed during treatment. Eight (17.0%) patients experienced at least one grade 3/4 AE and two patients discontinued treatment due to AEs. The common AEs (incidence ≥15%) were hand-foot syndrome (n=14, 29.8%), diarrhea (n=13, 27.7%), elevated aspartate transferase (n=11, 23.4%), and fatigue (n=8, 17.0%) (Table 3).
Table 3
| Variables | Total (n=47), n (%) |
|---|---|
| Postoperative complications | |
| Nausea | 21 (44.7) |
| Pain | 28 (59.6) |
| Vomiting | 8 (17.0) |
| Fever | 10 (21.3) |
| Adverse events | |
| Grade 3/4 adverse events | 8 (17.0) |
| Hand-foot syndrome | 14 (29.8) |
| Gastrointestinal ulcers | 7 (14.9) |
| Diarrhea | 13 (27.7) |
| Nausea | 7 (14.9) |
| Vomiting | 3 (6.4) |
| Anemia | 6 (12.8) |
| Leukopenia | 5 (10.6) |
| Cytopenia | 5 (10.6) |
| Bilirubinemia | 6 (12.8) |
| Elevated aspartate transferase | 11 (23.4) |
| Fatigue | 8 (17.0) |
Discussion
This study is the first to investigate the efficacy and safety of the combination of GSMs-TACE and regorafenib in patients with advanced HCC who have progressed after first-line targeted therapy. The results showed that the combination of GSMs-TACE and regorafenib had a positive effect on PFS and OS in patients with unresectable HCC, and the adverse effects of the combination therapy were tolerable. In this study, GSMs-TACE combined with regorafenib was shown to be an acceptable treatment regimen for unresectable HCC patients after failure of first-line targeted therapy. Before receiving GSMs-TACE combined with regorafenib, all patients had received 1 or more local therapies, 44 (93.6%) had received TACE, 8 (17.0%) had received ablation, and 7 (14.9%) had received surgical treatment. Before treatment, 24 (51.1%) patients received sorafenib only, 15 (31.9%) patients received lenvatinib only, and 8 patients (17.0%) received lenvatinib followed by sorafenib.
In this study, GSMs-TACE and regorafenib were combined to treat patients who progressed after first-line therapy, with mPFS of 6.0 months (95% CI: 4.5–7.5 months) and mOS of 14.3 months (95% CI: 11.8–16.8 months). Several studies have shown that compared with TACE alone or targeted drug therapy alone, TACE combined with targeted drug therapy can significantly improve the survival of HCC patients. Choi et al. (25) found that the mOS of patients treated with sorafenib combined with TACE was longer than that of the monotherapy group (median OS: 8.9 vs. 5.9 months, P=0.009). A retrospective cohort study showed that the 1- and 2-year OS rates of patients treated with TACE combined with lenvatinib (88.4% and 79.8%, respectively) were significantly higher than those of patients treated with TACE alone (79.2% and 49.2%, respectively) (P=0.047) (26). The advantage of combination therapy (TACE combined with systemic therapy) may be associated with a longer PFS and OS than monotherapy. These results warrant further confirmation in future studies.
In this study, multivariable Cox regression analysis showed that although the effect of complete embolization on OS remains unclear, the prolongation of PFS by complete embolization was statistically significant (HR =2.271, 95% CI: 1.023 to 5.041, P=0.044). Our previous study showed progressive results for GSMs-TACE in BCLC stage B–C and large HCC (23,27). When liver function is not significantly reduced, GSMs-TACE can completely embolize the tumor blood supply artery, causing significant tumor necrosis after the operation, reduced tumor burden in a short time, and significantly prolonged survival of patients (23,28). The uncertainty of the impact of the degree of embolism on OS suggests that the degree of embolization during GSMs-TACE on OS needs to be explored in future clinical studies. Accurate preoperative assessment of liver function and complete embolization of the tumor supply artery when liver function permits may have a positive effect in slowing the progression of the disease.
The DCR in this study was 76.6%, which was similar to the real-word study by Han et al. (76.3%) (19), but higher than the cohort study by Terashima et al. (35.0%) (29). Although TACE alone can achieve the purpose of reducing tumor burden in the short term in the treatment of unresectable HCC, due to the rich tumor blood supply, complex feeding arteries, and poor arterial patency, residual tumor masses supporting vessels are often left after TACE (30,31). At the same time, the reason for promoting disease progression or metastasis is often due to the fact that local hypoxia induced by a single TACE further promotes the formation of tumor blood vessels and bypasses the blocked tumor supplying artery, so it cannot effectively prolong the OS of unresectable HCC (32,33). TKIs, such as VEGFR-1, VEGFR-2, VEGFR-3, tyrosine protein kinase (Tie-2) and other protein kinases, not only inhibit tumor angiogenesis by inhibiting the activity of multiple vascular endothelial growth factor receptors (VEGFR), but also exert a variety of anti-tumor effects by inhibiting a variety of kinases involved in tumor proliferation and the tumor microenvironment (31,34-36). However, for advanced unresectable HCC, it is difficult to achieve the purpose of complete local inactivation of the tumor (10,37). TACE combined with TKI can effectively prolong the PFS and OS of HCC patients, the main reason being that TKI can produce a synergistic effect with TACE to inhibit tumor progression and metastasis (38,39).
This study demonstrated that the initial dose of regorafenib could significantly affect the OS and PFS of patients, and an initial dose ≥120 mg/d could prolong the PFS and OS of patients compared with doses ≤80 mg/d. Regorafenib has been recommended by several guidelines as a second-line drug for targeted therapy of HCC, and is often used in HCC patients after the progression of first-line sorafenib or lenvatinib treatment (3,7). Regorafenib targets a wider range of kinases and has a stronger inhibitory effect on VEGFR-2, platelet-derived growth factor receptor (PDGFR)-β, fibroblast growth factor receptor (FGFR)-1, and c-kit. In addition, it can also exert a broader anti-angiogenic effect by inhibiting Tie-2 (19,36,40). To date, a number of clinical studies have been conducted on TACE combined with sorafenib or lenvatinib, but there are few studies on TACE combined with regorafenib in the treatment of advanced HCC with first-line targeted drugs. A retrospective study involving a total of 38 patients showed that factors independently associated with PFS and OS were the initial dose of 120 or 160 mg/d (HR =0.216, 95% CI: 0.061–0.765, P=0.018) (19).The results suggested that starting with the maximum oral dose of regorafenib tolerated by patients should be considered in clinical practice. Finally, patients with CR, PR, or SD have longer OS and PFS than those with PD.
There were no level 5 AEs events (deaths) during the study period. Adverse reactions after TACE are predictable. There were 8 cases (17.0%) of grade 3/4 AEs, and 2 patients stopped treatment due to AE, which may be due to the lack of active surveillance.
Since this was a retrospective analysis, retrospective bias for uncontrolled parameters is inevitable and the conclusions of this report must be interpreted with caution. At the same time, the sample size of this study was small, and the conclusions may not effectively reflect the overall characteristics of patients with targeted therapy failure with first-line drugs. Furthermore, the study was limited by the absence of a control group. Nevertheless, this study could provide valuable insight into the combination therapy of GSMs-TACE and regorafenib, which may be a useful basis for future trials.
Conclusions
This investigation demonstrated that GSMs-TACE combined with regorafenib is effective and safe in patients with unresectable HCC who failed first-line sorafenib and lenvatinib therapy. Future prospective large-scale studies are warranted to verify these results.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1170/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1170/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1170/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by ethics committee of Affiliated Zhongshan Hospital of Dalian University (No. 2021022-1). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hepatocellular carcinoma. Nat Rev Dis Primers 2021;7:7. [Crossref] [PubMed]
- Vogel A, Martinelli EESMO Guidelines Committee, et al. Updated treatment recommendations for hepatocellular carcinoma (HCC) from the ESMO Clinical Practice Guidelines. Ann Oncol 2021;32:801-5. [Crossref] [PubMed]
- Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers 2021;7:6. [Crossref] [PubMed]
- European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018;69:182-236. Erratum in: J Hepatol 2019 Apr;70(4):817. [Crossref]
- Gordan JD, Kennedy EB, Abou-Alfa GK, et al. Systemic Therapy for Advanced Hepatocellular Carcinoma: ASCO Guideline. J Clin Oncol 2020;38:4317-45. [Crossref] [PubMed]
- Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol 2022;76:681-93. [Crossref] [PubMed]
- Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018;67:358-80. [Crossref] [PubMed]
- Galle PR, Tovoli F, Foerster F, et al. The treatment of intermediate stage tumours beyond TACE: From surgery to systemic therapy. J Hepatol 2017;67:173-83. [Crossref] [PubMed]
- Abdelrahim M, Victor D, Esmail A, et al. Transarterial Chemoembolization (TACE) Plus Sorafenib Compared to TACE Alone in Transplant Recipients with Hepatocellular Carcinoma: An Institution Experience. Cancers (Basel) 2022;14:650. [Crossref] [PubMed]
- Llovet JM, De Baere T, Kulik L, et al. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 2021;18:293-313. [Crossref] [PubMed]
- Wu Y, Qi H, Cao F, et al. TACE-Sorafenib With Thermal Ablation Has Survival Benefits in Patients With Huge Unresectable Hepatocellular Carcinoma. Front Pharmacol 2020;11:1130. [Crossref] [PubMed]
- Zhou B, Yan Z, Liu R, et al. Prospective Study of Transcatheter Arterial Chemoembolization (TACE) with Ginsenoside Rg3 versus TACE Alone for the Treatment of Patients with Advanced Hepatocellular Carcinoma. Radiology 2016;280:630-9. [Crossref] [PubMed]
- Arai H, Battaglin F, Wang J, et al. Molecular insight of regorafenib treatment for colorectal cancer. Cancer Treat Rev 2019;81:101912. [Crossref] [PubMed]
- Llovet JM, Montal R, Sia D, et al. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol 2018;15:599-616. [Crossref] [PubMed]
- Huang A, Yang XR, Chung WY, et al. Targeted therapy for hepatocellular carcinoma. Signal Transduct Target Ther 2020;5:146. [Crossref] [PubMed]
- Morse MA, Sun W, Kim R, et al. The Role of Angiogenesis in Hepatocellular Carcinoma. Clin Cancer Res 2019;25:912-20. [Crossref] [PubMed]
- Granito A, Forgione A, Marinelli S, et al. Experience with regorafenib in the treatment of hepatocellular carcinoma. Therap Adv Gastroenterol 2021;14:17562848211016959. [Crossref] [PubMed]
- Ding X, Sun W, Li W, et al. Transarterial chemoembolization plus lenvatinib versus transarterial chemoembolization plus sorafenib as first-line treatment for hepatocellular carcinoma with portal vein tumor thrombus: A prospective randomized study. Cancer 2021;127:3782-93. [Crossref] [PubMed]
- Han Y, Cao G, Sun B, et al. Regorafenib combined with transarterial chemoembolization for unresectable hepatocellular carcinoma: a real-world study. BMC Gastroenterol 2021;21:393. [Crossref] [PubMed]
- Zhao GS, Liu S, Liu Y, et al. Clinical application of gelatin sponge microparticles combined with pirarubicin for hepatic transcatheter arterial chemoembolization in breast cancer liver metastasis treatment: results of a single-center long-term study. World J Surg Oncol 2021;19:249. [Crossref] [PubMed]
- Li C, Liu Y, Zhou J, et al. A retrospective analysis of the efficacy of microparticle-mediated chemoembolization in liver metastases arising from gastrointestinal tumors. J Cancer Res Ther 2017;13:642-6. [Crossref] [PubMed]
- Zhao GS, Li C, Liu Y, et al. 350-560 μm gelatin sponge particles combined with transcatheter arterial chemoembolization for the treatment of elderly hepatocellular carcinoma: The safety and efficacy. Medicine (Baltimore) 2017;96:e6629. [Crossref] [PubMed]
- Zhou J, Liu Y, Ren Z, et al. Transarterial chemoembolization with gelatin sponge microparticles for barcelona clinic liver cancer Stage C and large hepatocellular carcinoma: Initial clinical experience. J Cancer Res Ther 2017;13:767-72. [Crossref] [PubMed]
- Bruix J, Reig M, Sherman M. Evidence-Based Diagnosis, Staging, and Treatment of Patients With Hepatocellular Carcinoma. Gastroenterology 2016;150:835-53. [Crossref] [PubMed]
- Choi GH, Shim JH, Kim MJ, et al. Sorafenib alone versus sorafenib combined with transarterial chemoembolization for advanced-stage hepatocellular carcinoma: results of propensity score analyses. Radiology 2013;269:603-11. [Crossref] [PubMed]
- Fu Z, Li X, Zhong J, et al. Lenvatinib in combination with transarterial chemoembolization for treatment of unresectable hepatocellular carcinoma (uHCC): a retrospective controlled study. Hepatol Int 2021;15:663-75. [Crossref] [PubMed]
- Kamran AU, Liu Y, Li FE, et al. Transcatheter Arterial Chemoembolization With Gelatin Sponge Microparticles Treated for BCLC Stage B Hepatocellular Carcinoma: A Single Center Retrospective Study. Medicine (Baltimore) 2015;94:e2154. [Crossref] [PubMed]
- Ren Z, Yue Y, Zhang Y, et al. Changes in the Peripheral Blood Treg Cell Proportion in Hepatocellular Carcinoma Patients After Transarterial Chemoembolization With Microparticles. Front Immunol 2021;12:624789. [Crossref] [PubMed]
- Terashima T, Yamashita T, Takata N, et al. Safety and efficacy of sorafenib followed by regorafenib or lenvatinib in patients with hepatocellular carcinoma. Hepatol Res 2021;51:190-200. [Crossref] [PubMed]
- Kudo M, Ueshima K, Ikeda M, et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut 2020;69:1492-501. [Crossref] [PubMed]
- Abou-Elkacem L, Arns S, Brix G, et al. Regorafenib inhibits growth, angiogenesis, and metastasis in a highly aggressive, orthotopic colon cancer model. Mol Cancer Ther 2013;12:1322-31. [Crossref] [PubMed]
- Cao F, Zheng J, Luo J, et al. Treatment efficacy and safety of regorafenib plus drug-eluting beads-transarterial chemoembolization versus regorafenib monotherapy in colorectal cancer liver metastasis patients who fail standard treatment regimens. J Cancer Res Clin Oncol 2021;147:2993-3002. [Crossref] [PubMed]
- Martin SP, Fako V, Dang H, et al. PKM2 inhibition may reverse therapeutic resistance to transarterial chemoembolization in hepatocellular carcinoma. J Exp Clin Cancer Res 2020;39:99. [Crossref] [PubMed]
- Wilhelm SM, Dumas J, Adnane L, et al. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer 2011;129:245-55. [Crossref] [PubMed]
- Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;389:56-66. [Crossref] [PubMed]
- Heo YA, Syed YY. Regorafenib: A Review in Hepatocellular Carcinoma. Drugs 2018;78:951-8. [Crossref] [PubMed]
- Raoul JL, Forner A, Bolondi L, et al. Updated use of TACE for hepatocellular carcinoma treatment: How and when to use it based on clinical evidence. Cancer Treat Rev 2019;72:28-36. [Crossref] [PubMed]
- Lencioni R, Llovet JM, Han G, et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: The SPACE trial. J Hepatol 2016;64:1090-8. [Crossref] [PubMed]
- Kudo M. A New Treatment Option for Intermediate-Stage Hepatocellular Carcinoma with High Tumor Burden: Initial Lenvatinib Therapy with Subsequent Selective TACE. Liver Cancer 2019;8:299-311. [Crossref] [PubMed]
- Wang W, Tsuchiya K, Kurosaki M, et al. Sorafenib-Regorafenib Sequential Therapy in Japanese Patients with Unresectable Hepatocellular Carcinoma-Relative Dose Intensity and Post-Regorafenib Therapies in Real World Practice. Cancers (Basel) 2019;11:1517. [Crossref] [PubMed]
(English Language Editor: J. Teoh)



