A study of gene variation in All-RAS wild-type metastatic colorectal cancer and its correlation with cetuximab
Highlight box
Key findings
• The PFS and OS of mCRC patients with all-RAS wild-type and no combined mutations treated with cetuximab were not better than those of patients with combined mutations.
What is known and what is new?
• It is well known that the RAS gene in mCRC is a standard biomarker for predicting first-line anti-EGFR therapy.
• We innovatively studied the influence of co-mutated genes on the efficacy of cetuximab by grouping tumor suppressor genes and oncogenic driver genes.
What is the implication, and what should change now?
• Alternative treatment strategies should be considered for mCRC patients with multiple oncogenic driver gene variants, even those genetically tested and determined to have the all-RAS wild-type, and all patients should undergo tumor-tissue based NGS testing at the baseline to determine if they would benefit from cetuximab monotherapy or combination therapy.
Introduction
Anti-epidermal growth factor receptor (EGFR) monoclonal antibodies (mAbs) (i.e., cetuximab and panitumumab) have been approved for use in combination with cytotoxic chemotherapy (1) for the first-line treatment of metastatic colorectal cancer (mCRC), and as a monotherapy or combination therapy for later-line treatments (2). Cetuximab is a chimeric mouse-human immunoglobulin G mAb that can bind to the extracellular domain of EGFR and induces the downregulation of proto-oncogene signaling. Cetuximab also can blinding to natural killer cells may trigger an immune-mediated antitumor response, leading to antibody-dependent cell-mediated cytotoxicity (3). It is well known that the RAS gene (KRAS/NRAS) in mCRC is a standard biomarker for predicting first-line anti-EGFR therapy. Even in patients with all-RAS wild-type mCRC, the efficacy of cetuximab differs, and it is unclear whether combined variants other than the RAS gene affects the efficacy.
Some retrospective studies have been conducted on the survival benefits of cetuximab in mCRC patients with different genetic variants; however, most studies have examined a single-gene variant (4). In the real world, there are many kinds of gene variants in mCRC patients, and multiple genetic variants often exist simultaneously. Different from the single gene variants in previous studies, this study innovatively studied the influence of co-mutated genes on the efficacy of cetuximab by grouping tumor suppressor genes and oncogenic driver genes. To extend understandings of the efficacy and influencing factors of cetuximab in treating all-RAS wild-type mCRC patients with different gene variant types, this study sought to retrospectively analyze the gene variants and clinical characteristics of all-RAS wild-type patients with mCRC, and the different prognosis of cetuximab in all-RAS wild-type patients, all-RAS wild-type patients with tumor suppressor gene mutations, and all-RAS wild-type patients with oncogenic driver gene mutations. A stratified study was also conducted to examine left-sided CRC and local interventions. Additionally, we searched for prognostic-related gene variant signatures to predict the efficacy of cetuximab in treating mCRC. We present the following article in accordance with the REMARK reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1237/rc).
Methods
Study design and participants
The data of patients with mCRC treated with cetuximab at the Oncology Department of The First Affiliated Hospital of Soochow University and The Second Affiliated Hospital of Soochow University from August 2016 to December 2020 were collected. Patients were considered eligible for the trial if they met the following inclusion criteria: (I) had histologically confirmed stage IV colorectal adenocarcinoma (according to the 8th UICC/AJCC TNM Staging System); (II) had the all-RAS gene wild-type as detected by next-generation sequencing (NGS) technology; (III) had undergone 4 cycles of cetuximab and at least 1 radiographic evaluation; and (IV) had a World Health Organization performance status of 0–2 before the start of the trial. At the baseline, patients had to have at least 1 lesion (with a diameter of more than 10 mm in the non-lymph-node lesions, or a short axis >15 mm in the lymph-node lesions) that had not been previously irradiated, that could be measured by computed tomography (CT) or magnetic resonance imaging (MRI), and that was suitable for repeated measurement. Patients were excluded from the study if they met any of the following main exclusion criteria: (I) had 2 or more primary tumors; (II) had a pathological type of squamous cell carcinoma, adenosquamous carcinoma, or another pathological type other than adenocarcinoma; and/or (III) had incomplete case information.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics committee of The First Affiliated Hospital of Soochow University (No. 2022-482) and ethics committee of The Second Affiliated Hospital of Soochow University (No. LK-2020-071-02). Informed consent was taken from all individual participants.
Procedures
The eligible patients received 400 mg/m2 of cetuximab (Erbitux, Merck KGaA, Germany) for the first week, followed by 250 mg/m2 of cetuximab weekly or 500 mg/m2 d1 of cetuximab fortnightly, by intravenous infusion of targeted therapy. Patients received the cetuximab in combination with the following treatment modalities: systemic cytotoxic chemotherapy (mFolfox6, or FOLFIRI), and local treatment (stereotactic body radiation therapy, stereotactic radio surgery, intensity modulated radio therapy, surgical excision, thermal ablation therapy, or transcatheter arterial chemoembolization). To determine the treatment effects, the enrolled patients were evaluated every 2–3 months during the follow-up period using CT, MRI, and positron emission tomography-CT as assessment methods and the standard response evaluation criteria in solid tumors (RECIST, version 1.1). Follow-up included obtaining survival information by telephone or at an outpatient clinic. The study had a cut-off date of January 31, 2021.
All the patients’ tumor tissue deoxyribonucleic acid (DNA) was sequenced using the NGS method at a depth of 1,000 for tissue and 6,000 for circulating-tumor DNA. With reference to the AMP/ASCO/CAP guidelines and databases, such as oncoKB, the variant genes were classified into the following categories based on the level of evidence of drug sensitivity: Class I, variants with clear clinical significance; Class II, variants with potential clinical significance; Class III, variants with no corresponding recommended drug use and possibly some clinical significance; and Class IV, other variants. The patients in this study mainly had genes in categories I, II, and III. The patients were divided into 3 groups according to the presence or absence of additional gene aberrations. Group A comprised all wild-type mCRC patients, group B comprised mCRC patients with concurrent all-RAS wild-type and mutations in tumor-suppressor genes (i.e., TP53, APC, PTEN, BRCA2, and SMAD4), and group C comprised mCRC patients with multiple alterations in oncogenic drivers (i.e., ERBB2, BRAF, PIK3CA, and RET) and all-RAS wild-type patients, irrespective of tumor-suppressor gene aberrances.
Outcomes
The primary outcome was progression-free survival (PFS), which was defined as the time the patient started taking the study drug until either objective disease progression (as assessed by an investigator using RECIST version 1.1) or death from any cause. The secondary outcomes were overall survival (OS), the objective response rate (ORR), and the disease control rate (DCR). OS was defined as the time the patient started taking the study until death from any cause. The ORR was defined as the percentage of patients with a confirmed complete response (CR) or partial response (PR) according to RECIST version 1.1. The DCR was defined as the percentage of patients who achieved disease control (i.e., CR, PR, or stable disease according to RECIST version 1.1) at 8 weeks or more after screening.
Statistical analysis
The data were analyzed statistically using SPSS 25.0 software, and the patients included in the study were analyzed for gene mutation status, and the mutation rate of each gene was expressed as a percentage. The distributions of the respective clinical characteristics were compared among the 3 groups using the chi-square test. The Kaplan-Meier method was used for the survival analysis and to plot the survival curves for PFS and OS, and the Log-rank method was used to compare the survival differences among groups A, B, and C. A P value <0.05 was considered statistically significant. In the multifactor analysis, the Cox regression model was used to identify which of the clinical characteristics were independent factors affecting PFS.
Results
An overview of the patient’s genetic variation
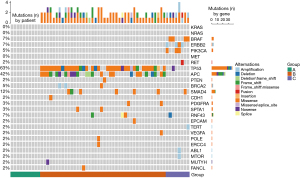
Group A comprised all-wild-type patients without other mutations (n=10), group B comprised all-RAS wild-type patients with tumor-suppressor genes (including TP53, APC, PTEN, BRCA2, and SMAD4) mutations (n=42), and group C comprised all-RAS wild-type patients with oncogenic driver genes (including ERBB2, BRAF, PIK3CA, and RET) alterations (n=8). The specific gene distributions are shown in Figure 1.
A total of 60 patients with mCRC were included in this retrospective study. Among the patients, 50 carried genetic mutations, among which, 40 (80.0%) had polygenic mutations. Notably, 33 (66%) patients had TP53 combined with other gene variants, among which 22 patients had APC, making it the most common combined gene variant. For further details, see Tables 1,2.
Table 1
| Mutant genes | Number of mutations | Mutation rate (%) |
|---|---|---|
| TP53 | 38 | 63.3 |
| APC | 25 | 41.7 |
| SMAD4 | 7 | 11.7 |
| ERBB2 | 4 | 6.7 |
| BRAF | 4 | 6.7 |
| RNF43 | 4 | 6.7 |
| PIK3CA | 3 | 5.0 |
| BRCA2 | 3 | 5.0 |
| PDGFRA | 2 | 3.3 |
| SPTA1 | 2 | 3.3 |
| RET | 1 | 1.7 |
| PTEN | 1 | 1.7 |
| CDH1 | 1 | 1.7 |
| ERCC4 | 1 | 1.7 |
| EPCAM | 1 | 1.7 |
| TERT | 1 | 1.7 |
| VEGFA | 1 | 1.7 |
| POLE | 1 | 1.7 |
| ABL1 | 1 | 1.7 |
| MTOR | 1 | 1.7 |
| MUTYH | 1 | 1.7 |
| FANCL | 1 | 1.7 |
| ERBB4 | 1 | 1.7 |
| DPYD | 1 | 1.7 |
Table 2
| Mutant genes | Number | % | |
|---|---|---|---|
| TP53 and tumor-suppressor mutations | TP53+APC | 14 | 28 |
| TP53+APC+BRCA2 | 2 | 4 | |
| TP53+APC+SMAD4 | 2 | 4 | |
| TP53+APC+CDH1 | 1 | 2 | |
| TP53+APC+ MUTYH | 1 | 2 | |
| TP53+APC+SPTA1 | 1 | 2 | |
| TP53+APC+RNF43 | 1 | 2 | |
| TP53+ SMAD4 | 3 | 6 | |
| TP53+ SMAD4+EPCAM | 1 | 2 | |
| TP53 and oncogenic driver mutations | TP53+ BRAF | 1 | 2 |
| TP53 and other mutations | TP53+RNF43 | 2 | 4 |
| TP53+PDGFRA | 1 | 2 | |
| TP53+FANCL+YAP1 | 1 | 2 | |
| TP53+ERBB4+FANCD2+POLE | 1 | 2 | |
| TP53+WT1+EPHB1+ESR1+KDR+TYR | 1 | 2 |
Relationship between genetic variation and clinical features
The demographic and baseline characteristics of the 60 patients in the full analysis set are summarized in Table 3.
Table 3
| Clinical feature | Participants (n=60) |
|---|---|
| Age (years) | |
| ≤60 | 30 (50.0%) |
| >60 | 30 (50.0%) |
| Gender | |
| Men | 39 (65.0%) |
| Women | 21 (35.0%) |
| Degree of tissue differentiation | |
| Low | 4 (6.7%) |
| Low-medium | 8 (13.3%) |
| Medium | 33 (55.0%) |
| Unknown | 15 (25.0%) |
| Primary lesion site | |
| Left | 49 (81.7%) |
| Right | 11 (18.3%) |
| Number of transferred organs | |
| Single | 32 (53.3%) |
| Multiple | 28 (46.7%) |
| Transfer type | |
| Simultaneous | 38 (63.3%) |
| Heterochronous | 22 (36.7%) |
| Local intervention of metastatic site | |
| No | 32 (53.3%) |
| Yes | 28 (46.7%) |
The relationship between different gene mutations and clinical features is shown in Table 4. Mutations in the TP53 (86.8%), APC (84.0%), and SMAD4 (85.7%) genes were the most common in left-sided mCRC.
Table 4
| Clinical feature | TP53 (n=38) | APC (n=25) | SMAD4 (n=7) | BRAF (n=4) | ERBB2 (n=4) | PIK3CA (n=3) |
|---|---|---|---|---|---|---|
| Gender | ||||||
| Men | 26 (68.4%) | 20 (80.0%) | 3 (42.9%) | 1 (25.0%) | 2 (50.0%) | 2 (66.7%) |
| Women | 12 (31.6%) | 5 (20.0%) | 4 (57.1%) | 3 (75.0%) | 2 (50.0%) | 1 (33.3%) |
| Primary lesion site | ||||||
| Left | 33(86.8%) | 21 (84.0%) | 6 (85.7%) | 3 (75.0%) | 3 (75.0%) | 1 (33.3%) |
| Right | 5 (13.2%) | 4 (16.0%) | 1 (14.3%) | 1 (25.0%) | 1 (25.0%) | 2 (66.7%) |
| Number of transferred organs | ||||||
| Single | 18 (47.4%) | 14 (56.0%) | 2 (28.6%) | 1 (25.0%) | 1 (25.0%) | 1 (33.3%) |
| Multiple | 20 (52.6%) | 11 (44.0%) | 5 (71.4%) | 3 (75.0%) | 3 (75.0%) | 2 (66.7%) |
| Transfer Type | ||||||
| Simultaneous | 23 (60.5%) | 16 (64.0%) | 3 (42.9%) | 4 (100.0%) | 1 (25.0%) | 1 (33.3%) |
| Heterochronous | 15 (39.5%) | 9 (36.0%) | 4 (57.1%) | 0 (0.0%) | 3 (75.0%) | 2 (66.7%) |
| Liver metastasis | ||||||
| No | 18 (47.4%) | 8 (32.0%) | 5 (71.4%) | 1 (25.0%) | 4 (100.0%) | 3 (100.0%) |
| Yes | 20 (52.6%) | 17 (68.0%) | 2 (28.6%) | 3 (75.0%) | 0 (0.0%) | 0 (0.0%) |
| Lung metastasis | ||||||
| No | 29 (76.3%) | 20 (80.0%) | 4 (57.1%) | 2 (50.0%) | 2 (50.0%) | 2 (66.7%) |
| Yes | 9 (23.7%) | 5 (20.0%) | 3 (42.9%) | 2 (50.0%) | 2 (50.0%) | 1 (33.3%) |
The chi-square test was used to detect differences between groups A, B, and C at the level of each clinical characteristic; however, the P values among the groups were >0.05; thus, there were no statistically significant differences between the groups, indicating that the factors were balanced and comparable among the 3 groups (see Table 5 for further details).
Table 5
| Clinical feature | Group A (n=10) | Group B (n=42) | Group C (n=8) | P |
|---|---|---|---|---|
| Gender | 0.180 | |||
| Women | 4 (40.0%) | 12 (28.6%) | 5 (62.5%) | |
| Men | 6 (60.0%) | 30 (71.4%) | 3 (37.5%) | |
| Age, mean (SD) | 51.8 (12.7) | 60.0 (13.0) | 60.2 (10.3) | 0.176 |
| Degree of tissue differentiation | 0.616 | |||
| Low | 1 (10.0%) | 2 (4.8%) | 1 (12.5%) | |
| Low-medium | 2 (20.0%) | 5 (11.9%) | 1 (12.5%) | |
| Medium | 6 (60.0%) | 22 (52.4%) | 5 (62.5%) | |
| Unknown | 1 (10.0%) | 13 (31.0%) | 1 (12.5%) | |
| Primary lesion site | 0.155 | |||
| Left | 6 (60.0%) | 36 (85.7%) | 7 (87.5%) | |
| Right | 4 (40.0%) | 6 (14.3%) | 1 (12.5%) | |
| Transfer type | 0.915 | |||
| Simultaneous | 7 (70.0%) | 26 (61.9%) | 5 (62.5%) | |
| Heterochronous | 3 (30.0%) | 16 (38.1%) | 3 (37.5%) | |
| Number of transferred organs | 0.185 | |||
| Single | 7 (70.0%) | 23 (54.8%) | 2 (25.0%) | |
| Multiple | 3 (30.0%) | 19 (45.2%) | 6 (75.0%) | |
| Local intervention of metastatic site | 0.851 | |||
| No | 5 (50.0%) | 22 (52.4%) | 5 (62.5%) | |
| Yes | 5 (50.0%) | 20 (47.6%) | 3 (37.5%) | |
Group A: all-wild-type patients without other mutations; Group B: all-RAS wild-type patients with tumor-suppressor genes (including TP53, APC, PTEN, BRCA2, and SMAD4) mutations; Group C: comprised all-RAS wild-type patients with oncogenic driver genes (including ERBB2, BRAF, PIK3CA, and RET) alterations.
Effect of cetuximab on PFS and OS in the treatment of mCRC with different gene variants
At the time of the data cut-off date (i.e., January 31, 2021), 39 patients had progressive disease or had died; however, the OS data were not yet available. The median follow-up time was 14.5 months (range, 2.0–50.0 months).
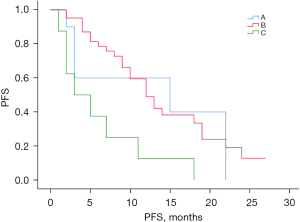
Figure 2 shows the comparison of the PFS curves for groups A, B, and C. The median PFS time for the total sample was 12.0 months [95% confidence interval (CI): 8.95–15.05 months], and the median PFS times for groups A, B, and C were 15.0 months (95% CI: 0.00–37.72 months), 12.0 months (95% CI: 9.01–14.99 months), and 3.0 months (95% CI: 0.00–7.16 months), respectively. PFS differed significantly among the 3 groups (χ2=9.965, P=0.007). However, the crossover in the survival curves suggested the possible existence of uncorrected confounders. Based on the univariate results, we fit a Cox proportional risk regression model with gender as a stratified variable to correct for the effect of each confounding factor and plotted the survival analysis results (see Figures 3,4). The results of the Cox survival analysis showed that there was no statistically significant difference in the PFS time of patients in group A compared to the PFS times of patients in groups B and C (P=0.882 and 0.071).
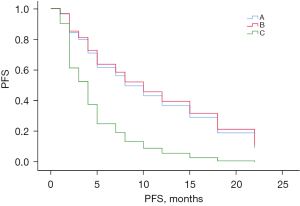
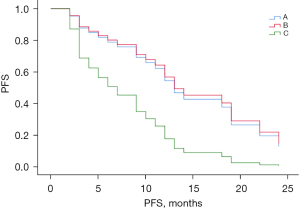
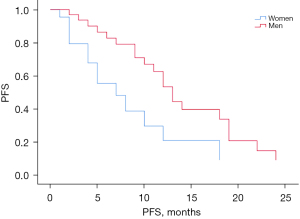
Figure 5 shows the comparison of the survival curves of PFS in women and men in groups B and C. The results showed that the PFS times in groups B and C differed significantly (P=0.011). In conclusion, while the results of the median PFS comparison showed that patients in group A had significantly prolonged PFS compared to groups B and C, and patients in group B had significantly prolonged PFS compared to group C, after adjusting for confounding factors and the statistical analysis, only group B had significantly prolonged PFS compared to group C.
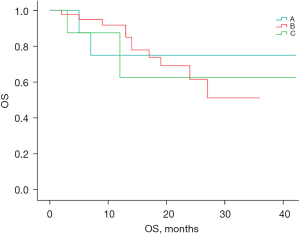
Of the 60 patients enrolled, 18 were confirmed to have died by the follow-up cut-off date. Figure 6 shows the OS survival curves of groups A, B, and C. There was no statistically significant difference in OS between the 3 groups (P=0.998). However, due to the short follow-up period of this study, the median number of OS events had not yet been reached.
Cetuximab in left hemisphere mCRC with different gene variant types
There were 49 patients with mCRC whose primary tumor location was on the left side, 6 of whom were in group A, 36 of whom were in group B, and 7 of whom were in group C. The median PFS time in patients with left-sided mCRC was 12.0 months (95% CI: 9.85–14.15 months). The median PFS times were 3.0, 13.0, and 3.0 months for groups A, B, and C, respectively, in patients with left-sided mCRC, and the differences among the 3 groups were significant (see Figure 7, P=0.009). However, due to the small number of patients in group A included and not included in the post-statistics, the results only showed that PFS was significantly longer in group B patients compared to group C patients with left-sided mCRC. As Figure 8 shows, there was no statistically significant difference in the OS between groups A, B, and C in patients with left-sided mCRC (P=0.945).
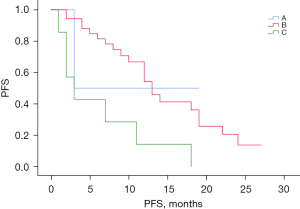
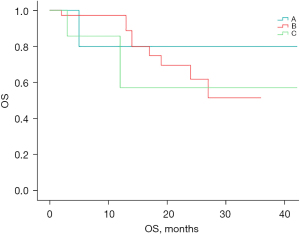
Effect of local intervention
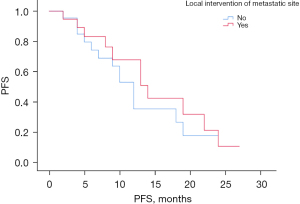
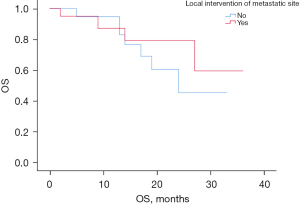
There were 42 patients in group B, including 20 patients who underwent local intervention and 22 patients who did not undergo local intervention. Figure 9 suggests that there was no statistical difference in PFS between the local intervention and non-local intervention groups in the Group B patients (P=0.55). The local intervention mCRC patients had a median PFS time of 14.0 months (95% CI: 8.64–19.36 months) and the non-local intervention mCRC patients had a median PFS time of 12.0 months (95% CI: 9.65–14.35 months). Figure 10 suggests that there was also no statistically significant difference in the OS between the localized intervention and non-localized intervention groups in Group B patients (P=0.433).
Univariate and multivariate analysis
Based on the results of the univariate analysis (see Table 6), none of the clinical characteristic factors had a significant effect on PFS (P<0.05). Among the factors, different gene mutation types had the largest effect on PFS (0.05≤P<1). Given the limitations of the univariate analysis, gene mutation types was included in the multifactorial analysis in this study.
Table 6
| B | SE | Wald | P | HR | |
|---|---|---|---|---|---|
| Group | |||||
| A | Ref | ||||
| B | –0.161 | 0.459 | 0.123 | 0.726 | 0.851 |
| C | 1.047 | 0.550 | 3.622 | 0.057 | 2.850 |
| Gender | |||||
| Women | Ref | ||||
| Men | –0.459 | 0.329 | 1.945 | 0.163 | 0.632 |
| Age | 0.004 | 0.014 | 0.082 | 0.774 | 1.004 |
| Degree of tissue differentiation | |||||
| Low | Ref | ||||
| Low-medium | 0.116 | 1.125 | 0.011 | 0.918 | 1.123 |
| Medium | 0.426 | 1.030 | 0.171 | 0.679 | 1.532 |
| Unknown | 0.346 | 1.061 | 0.106 | 0.745 | 1.413 |
| Primary lesion site | |||||
| Left | Ref | ||||
| Right | 0.304 | 0.400 | 0.580 | 0.446 | 1.356 |
| Transfer type | |||||
| Simultaneous | Ref | ||||
| Heterochronous | 0.571 | 0.335 | 2.902 | 0.088 | 1.770 |
| Number of transferred organs | |||||
| Single | Ref | ||||
| Multiple | 0.526 | 0.328 | 2.570 | 0.109 | 1.693 |
| Local intervention of metastatic site | |||||
| No | Ref | ||||
| Yes | –0.365 | 0.327 | 1.247 | 0.264 | 0.695 |
As Tables 6,7 show, the final screened model included only the subgroup variables with different mutation types. Only subgroups B and C differed significantly in terms of their effects on patients PFS (P=0.004).
Table 7
| Group | B | SE | Wald | P | HR (95% CI) |
|---|---|---|---|---|---|
| Group 1 | |||||
| A | Ref | ||||
| B | –0.161 | 0.459 | 0.123 | 0.726 | 0.851 (0.346–2.092) |
| C | 1.047 | 0.550 | 3.622 | 0.057 | 2.850 (0.969–8.378) |
| Group 2 | |||||
| C | Ref | ||||
| A | –1.047 | 0.550 | 3.622 | 0.057 | 0.351 (0.119–1.032) |
| B | –1.208 | 0.417 | 8.382 | 0.004 | 0.299 (0.132–0.677) |
Discussion
Our survival analysis showed that the RAS wild-type mCRC patients with the tumor suppressor gene mutations who received cetuximab combined with chemotherapy had significantly longer PFS than those with all-RAS wild-type mCRC combined with the oncogenic driver gene variants and those with all-RAS wild-type mCRC no combined with the gene variants. Wild-type mCRC patients without genetic variants did not outperform the other 2 groups in terms of either PFS or OS. The BENEFIT trial examined the efficacy of gefitinib in patients with advanced non-small cell lung cancer (NSCLC) with EGFR mutations combined with different genetic variants in 3 groups based on the NGS results of the patients. The results showed that the median PFS time of patients with only the EGFR mutation treated with gefitinib was significantly longer than that of patients with the EGFR mutation combined with other gene variants, and the median PFS of patients with the EGFR mutation combined with tumor suppressor gene variants was significantly longer than that of patients with the EGFR mutation combined with the oncogenic driver gene variants (5). Thus, this study divided the all-RAS wild-type mCRC patients into the following 3 groups: (I) patients without combined gene variants; (II) patients with combined tumor suppressive gene variants (including TP53, APC, PTEN, BRCA2, and SMAD4); and (III) patients with combined oncogenic driver gene variants (including HER2/ERBB2, BRAF, PIK3CA, and RET). A Kaplan-Meier analysis of the enrolled mCRC patients was performed to clarify the indication population for treatment with cetuximab. In this study, it was observed that treatment with cetuximab in patients with all-RAS wild-type and no combined mutation was not superior to that of patients with a combined mutation in terms of PFS and OS. Our results differ slightly to those for NSCLC.
Oncogenic driver gene
In a series of previous retrospective studies or single-arm phase-II studies, other genetic alterations in the EGFR signaling pathway have been found to be associated with resistance to EGFR mAbs, and the activation of intracellular signaling pathways downstream of EGFR (including the RAS-RAF-MAPK, PI3K-PTEN-AKT, and JAK/STAT signaling pathways) has been shown to be an important mechanism for generating resistance to EGFR mAbs (6-10). Changes in any of the components may lead to the constitutive activation of the EGFR, consequent intracellular signaling, and ultimately drug resistance (11).
As an important member of the oncogenic driver genes, BRAF mutations are present in 8% to 12% of mCRC cases, and every clinical trial conducted to date and real-world data have shown that the prognosis of mCRC patients with BRAF gene mutations is poor, especially, for those with V600E mutations whose mCRC prognosis is even worse (12). Whether EGFR is blocked or not, BRAF V600E mutations can still cause continuous activation of downstream signals, leading to tumor cell proliferation and survival (13,14).
Further, BRAF mutations only occur in tumors that do not carry RAS mutations. In recent years, in addition to RAS mutations in the tumor, BRAF mutation status is also important to consider before administering anti-EGFR therapy. ERBB2, an oncogenic driver gene in the EGFR signaling pathway, is a transmembrane glycoprotein with receptor tyrosine kinase activity. ERBB2 amplification can bypass EGFR signaling and activate the MEK-ERK cascade response. ERBB2 amplification has been observed in some patients with all-RAS and BRAF wild-type mCRC who are insensitive to cetuximab treatment (15). Through prospective randomized trial in mice, it was finally found that genotype-response correlation showed that HER2 was specifically amplified in cetuximab resistant and KRAS/NRAS/BRAF/PIK3CA wild-type cases (16). Similarly, Yonesaka et al. (17) observed that ERRB2 signaling was activated in mCRC patients who showed resistance to cetuximab treatment, activation depending on ERBB2 amplification or heregulin upregulation. Mutations in another oncogenic driver gene, PIK3CA, occur in approximately 10–18% of patients with CRC, mainly in exon 9 (E542K and E545K) and exon 20 (H1047R) (18). In 2005, PIK3CA mutations in the PI3K-PTEN-AKT signaling pathway were identified as possible predictors of anti-EGFR resistance in RAS wild-type mCRC (19). Since then, several systematic review studies have confirmed that PIK3CA mutations serve as predictors of anti-EGFR resistance in RAS wild-type mCRC (20-23). Thus, previous research has shown that mutations in oncogenic driver genes (BRAF, ERBB2, and PIK3CA) lead to cetuximab resistance, which is consistent with our findings that the survival benefit was reduced in the all-RAS wild-type group with the oncogenic driver gene mutation treated with cetuximab.
Tumor suppressor gene
The tumor suppressor gene PTEN is also a member of the EGFR signaling pathway; however, some studies have shown that the loss of PTEN may be related to anti-EGFR resistance; however, the role of PTEN loss in mCRC still unclear. Several studies have reported inconsistent results on the effect of PTEN loss against EGFR resistance (24). Further studies and prospective large randomized clinical trials need to be conducted to confirm the role of PTEN in anti-EGFR treatment resistance. Additionally, as only 1 patient carried the PTEN loss mutation in the RAS wild-type group with tumor-suppressor mutations in this study, the impact on the OS is minor (25,26).
Primary tumor location
In terms of PFS, the subgroup analysis showed cetuximab had a better effect on the patients with the all-RAS wild-type with the tumor suppressor variants in the left half of mCRC than that of patients with the all-RAS wild-type with oncogenic driver variants. The location of the primary tumor is very important in mCRC (27). A subgroup analysis of GALGB/SWOG 80405 showed (28) that primary tumor location was an independent prognostic factor for mCRC. The embryological origin, anatomy, and clinical manifestations of left and right CRC differ. Studies have shown that right CRC does not benefit from anti-EGFR therapy (in terms of both PFS and OS) in the context of first-line treatment of mCRC (29,30). Compared to left CRC, right CRC is more likely to contain downstream or bypass drivers of EGFR (e.g., RAS, BRAF, and PIK3CA mutations, hypermethylation, HER2 overexpression, and reduced EGFR ligand expression), leading to anti-EGFR resistance. However, even after eliminating these currently known molecular events, the effect of tumor site on efficacy cannot be fully explained. The difference between the left and right CRC is significant, and comprehensive analyses of multiple randomized studies have also confirmed this difference (29,31). The 2017 update to the NCCN guidelines limits cetuximab first-line therapy to those with primary tumors on the left side. Thus, this study conducted a subgroup analysis of the efficacy of cetuximab in patients with different genetic variants in left RAS wild-type mCRC.
Local intervention
Several previous studies have suggested an improvement in the prognosis of mCRC patients with local interventions, especially the resection of hepatic metastasis. However, few trials have investigated the effects of local intervention on the efficacy of cetuximab. The present study found that local intervention failed to improve the efficacy (including the PFS and OS) of cetuximab in mCRC patients with all-RAS wild-type combined with the tumor-suppressor variant. Further confirmations of these findings in large clinical studies are needed to inform the future clinical application of cetuximab.
In this study, the univariate and multivariate analyses showed that RAS wild-type mCRC combined with the tumor suppressor gene or oncogenic driver gene variant was an independent risk factor for PFS. Recent research in China has shown that the primary tumor site is an independent factor influencing the prognosis of PFS in patients with KRAS wild-type mCRC treated with cetuximab (P<0.05). We did not find any correlation between the primary tumor site and prognosis; however, this may have been due to the small number of cases with primary tumors on the right side, and the large difference to the number of cases with primary tumors on the left.
In the present study, both the overall analysis and the subgroup analysis revealed no significant difference in the efficacy of the 3 groups of patients in terms of OS (P>0.05). This may be related to the short follow-up period of this study in which only 18 patients died (3/10 in group A, 11/42 in group B, and 4/8 in group C) and the median number of OS events not yet reached. Thus, a longer follow-up study needs to be conducted to determine whether there is a significant difference in OS between mCRC patients with different combined genetic variant types treated with cetuximab.
Acquired resistance mechanism
In this study, 39 (65.0%) patients showed eventual progression. Acquired resistance refers to the patients who are initially effective for treatment and finally progress. Clinical data suggest that the remission duration of patients who undergo anti-EGFR therapy is relatively short, with most tumors becoming refractory within 3–12 months (32). Thus, numerous mechanisms may contribute to patients’ acquired resistance to anti-EGFR therapy, including secondary changes in the RAS-RAF signaling pathway (33,34), the activation of the IGF-1R pathway (35), MET overexpression and amplification (36), HER2 amplification and HER3/4 ligand overexpression (16,17), EGFR S492R mutation (37,38), and altered VEGF signaling (39).
The first acquired resistance mechanism is the secondary alteration of the RAS-RAF signaling pathway. RAS mutations play a crucial role in acquired resistance. About 50% of acquired resistance cases are due to secondary RAS mutations (33,34,40,41). The global Phase III ASPECT study used NGS to detect RAS mutations in ctDNA of patients treated with anti-EGFR therapy. The results showed that RAS mutations occurred in 32% of 164 patients whose baseline ctDNA was RAS wild type (42). Further, research has shown that alterations in these genes are likely due to the cloning of pre-existing drug-resistant cells.
The second acquired resistance mechanism is due to the activation of other growth factor receptor signaling pathways. For example, IGF-1R, MET (15), and HER2 (43) can bypass EGFR to activate EGFR downstream effectors and trigger subsequent intracellular signaling pathways, thereby inducing tumor cell proliferation and resistance to apoptosis. IGF-1R belongs to the transmembrane tyrosine kinase family and is activated upon binding to IGF-1 or IGF-2. Binding leads to activation downstream of the RAS-RAF-MAPK and PI3K-AKT pathways. Additional pre-clinical studies have shown that signaling via IGF-1R activation also leads to an increase in EGFR activation (44), resulting in acquired resistance to EGFR-targeted therapies (44,45). The MET gene leads to cell proliferation and survival via the activation of intracellular signaling cascades, including the PI3K-AKT, RAC1-CDC42, RAP1, and RAS-MAPK pathways (46). The interaction of EGFR-MET with MET pathway activation induced by TGF-α overexpression has been suggested as a possible mechanism for the acquired resistance to cetuximab in CRC (47). This was demonstrated in a study by Liska et al. (36) in 2011. Interestingly, further analysis showed that cetuximab also restored the effect through the pharmacological inhibition and silencing of MET. Further, both mechanisms (i.e., HER2 gene amplification and HER3/4 ligand heregulin overexpression) may lead to the sustained activation of ERK signaling, thus leading to secondary resistance to EGFR-targeted therapy (16,17). Several studies have shown that previously uncommon HER2 amplifying clones may amplify under the pressure of anti-EGFR therapy, leading to disease progression due to acquired drug resistance.
The EGFR S492R mutation is also a possible reason for the development of acquired resistance to EGFR-targeted therapy (37). The mutation reduces the affinity of the receptor for the ligand and interferes with the binding of cetuximab. It has not been detected in untreated patients in several studies (38). In contrast, the S492R mutation does not affect the action of panitumumab. Thus, panitumumab treatment appears to be a reasonable strategy for patients with S492R mutations who develop disease progression after treatment with cetuximab.
In addition, alterations in VEGF signaling may also lead to acquired resistance to EGFR-targeted therapies. Ciardiello et al. (48) showed that the high expression of VEGF in CRC cells is correlated with resistance to EGFR inhibitors. Bianco et al. (49) found higher levels of VEGF and VEGFR1 secretion in cetuximab-resistant cells compared to cetuximab-sensitive cells. Additionally, EGFR monoclonal resistant cells could be inhibited by VEGFR1 silencing or Vandetanib. These results suggest that combined VEGFR and EGFR inhibition restores patients’ sensitivity to anti-EGFR drugs and provides further evidence of the association between increased VEGF/VEGFR1 expression with resistance to anti-EGFR therapy.
Primary resistance mainly including changes of EGFR and EGFR ligands, RAS mutation, BRAF mutation, PTEN loss, activation of the PIK3CA/PTEN or JAK/STAT signaling pathways. These therapies could be used to reverse the resistance: new EGFR-targeted inhibitors (eg. GC1118, MM-151), a combination of multitargeted inhibitors, metabolic regulators, new cytotoxic drugs, modification or activation of immune cells, suppression of cancer-associated fibroblasts and anti-VEGFR agents (39).
Limitations
This study had a number of limitations: First, this study was retrospective, and unlike prospective studies, it could not control for various types of confounding factors; thus, the data may be biased. Second, while this study was conducted as a multicenter clinical study, the number of cases was small, the sample size of some of the subgroups after grouping was small, and the number of cases varied widely among subgroups; thus, the analysis of the results should be interpreted with caution, and the accuracy of the conclusions needs to be validated by large samples of evidence-based medicine. Third, due to objective constraints, the follow-up period of this study was short; thus, the OS endpoint was not met in most cases, and the study results may partially change with the extension of the follow-up period. PFS is the primary study endpoint of this study due to its ability to provide earlier results for analysis, which can be more accurately detected and attributed to the effect of the investigational treatment without being influenced by any subsequent treatment (50). However, for large clinical trials of advanced tumors, OS is the gold standard endpoint because it is easy to measure and accurate. Conversely, PFS lacks accepted consensus criteria, and there may be other measures that limit the survival benefits, which may affect the results.
Conclusions
In the real world, where there are multiple lines of treatment options for mCRC, the advent of NGS offers new possibilities for determining the prognosis of tumor patients, evaluating hyper-indicated targeted therapies for refractory cancers, and accelerating research on matched targeted therapies (51,52). The staging of CRC was correlated with the depth of tumor invasion, the number of lymph node metastasis and the presence of distant metastasis. Existing studies have shown that the prognosis of mCRC is related to the status of RAS and BRAF, lymph node metastasis or not, the time of metastasis, the number and size of metastasis, the general status of patients, complications and so on. In this study, patients with all-RAS wild-type mCRC were selected as the research objects, and the efficacy of cetuximab treatment in patients with all-RAS wild-type mCRC, all-RAS wild-type mCRC with tumor suppressor gene variant and all-RAS wild-type mCRC with oncogene driver gene variant were compared after excluding common prognostic factors. In the balance of other related factors, cetuximab treatment was shown to have a greater benefit in mCRC all-RAS wild-type patients with the tumor suppressive gene variant. Our findings provide a certain basis for the selection of treatment strategies for patients with mCRC in clinical practice. Notably, in the cetuximab treatment of all-RAS wild-type mCRC patients with tumor suppressor gene variants, the local intervention did not provide any survival benefits. Thus, local treatments should only be carefully administered to RAS wild-type patients with the tumor suppressor gene variant treated with cetuximab. Alternative treatment strategies should be considered for mCRC patients with multiple oncogenic driver gene variants, even those genetically tested and determined to have the all-RAS wild-type, and all patients should undergo tumor-tissue based NGS testing at the baseline to determine if they would benefit from cetuximab monotherapy or combination therapy.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1237/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1237/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1237/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics committee of The First Affiliated Hospital of Soochow University (No. 2022-482) and ethics committee of The Second Affiliated Hospital of Soochow University (No. LK-2020-071-02). Informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Douillard JY, Oliner KS, Siena S, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med 2013;369:1023-34. [Crossref] [PubMed]
- Peeters M, Price TJ, Cervantes A, et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol 2010;28:4706-13. [Crossref] [PubMed]
- Yarom N, Jonker DJ. The role of the epidermal growth factor receptor in the mechanism and treatment of colorectal cancer. Discov Med 2011;11:95-105. [PubMed]
- Zhu N, Fang X, Li D, et al. Identification and prognostic analysis of the cetuximab resistance-related gene REV1 in RAS wild-type metastatic colorectal cancer. Am J Cancer Res 2021;11:2769-81. [PubMed]
- Wang Z, Cheng Y, An T, et al. Detection of EGFR mutations in plasma circulating tumour DNA as a selection criterion for first-line gefitinib treatment in patients with advanced lung adenocarcinoma (BENEFIT): a phase 2, single-arm, multicentre clinical trial. Lancet Respir Med 2018;6:681-90. [Crossref] [PubMed]
- De Roock W, Claes B, Bernasconi D, et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol 2010;11:753-62. [Crossref] [PubMed]
- Mao C, Yang ZY, Hu XF, et al. PIK3CA exon 20 mutations as a potential biomarker for resistance to anti-EGFR monoclonal antibodies in KRAS wild-type metastatic colorectal cancer: a systematic review and meta-analysis. Ann Oncol 2012;23:1518-25. [Crossref] [PubMed]
- Jeong JH, Kim J, Hong YS, et al. HER2 Amplification and Cetuximab Efficacy in Patients With Metastatic Colorectal Cancer Harboring Wild-type RAS and BRAF. Clin Colorectal Cancer 2017;16:e147-52. [Crossref] [PubMed]
- Stahler A, Stintzing S, von Einem JC, et al. Single-nucleotide variants, tumour mutational burden and microsatellite instability in patients with metastatic colorectal cancer: Next-generation sequencing results of the FIRE-3 trial. Eur J Cancer 2020;137:250-9. [Crossref] [PubMed]
- Georgiou A, Stewart A, Vlachogiannis G, et al. A phospho-proteomic study of cetuximab resistance in KRAS/NRAS/BRAF(V600) wild-type colorectal cancer. Cell Oncol (Dordr) 2021;44:1197-206. [Crossref] [PubMed]
- Hsu HC, Thiam TK, Lu YJ, et al. Mutations of KRAS/NRAS/BRAF predict cetuximab resistance in metastatic colorectal cancer patients. Oncotarget 2016;7:22257-70. [Crossref] [PubMed]
- Sanz-Garcia E, Argiles G, Elez E, et al. BRAF mutant colorectal cancer: prognosis, treatment, and new perspectives. Ann Oncol 2017;28:2648-57. [Crossref] [PubMed]
- Zhao B, Wang L, Qiu H, et al. Mechanisms of resistance to anti-EGFR therapy in colorectal cancer. Oncotarget 2017;8:3980-4000. [Crossref] [PubMed]
- Chen K, Zhang Y, Qian L, et al. Emerging strategies to target RAS signaling in human cancer therapy. J Hematol Oncol 2021;14:116. [Crossref] [PubMed]
- Bardelli A, Corso S, Bertotti A, et al. Amplification of the MET receptor drives resistance to anti-EGFR therapies in colorectal cancer. Cancer Discov 2013;3:658-73. [Crossref] [PubMed]
- Bertotti A, Migliardi G, Galimi F, et al. A molecularly annotated platform of patient-derived xenografts ("xenopatients") identifies HER2 as an effective therapeutic target in cetuximab-resistant colorectal cancer. Cancer Discov 2011;1:508-23. [Crossref] [PubMed]
- Yonesaka K, Zejnullahu K, Okamoto I, et al. Activation of ERBB2 signaling causes resistance to the EGFR-directed therapeutic antibody cetuximab. Sci Transl Med 2011;3:99ra86. [Crossref] [PubMed]
- Lièvre A, Bachet JB, Le Corre D, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res 2006;66:3992-5. [Crossref] [PubMed]
- Moroni M, Veronese S, Benvenuti S, et al. Gene copy number for epidermal growth factor receptor (EGFR) and clinical response to antiEGFR treatment in colorectal cancer: a cohort study. Lancet Oncol 2005;6:279-86. [Crossref] [PubMed]
- Wong NS, Fernando NH, Nixon AB, et al. A phase II study of capecitabine, oxaliplatin, bevacizumab and cetuximab in the treatment of metastatic colorectal cancer. Anticancer Res 2011;31:255-61. [PubMed]
- Saridaki Z, Tzardi M, Papadaki C, et al. Impact of KRAS, BRAF, PIK3CA mutations, PTEN, AREG, EREG expression and skin rash in >/= 2 line cetuximab-based therapy of colorectal cancer patients. PLoS One 2011;6:e15980. [Crossref] [PubMed]
- Tol J, Dijkstra JR, Klomp M, et al. Markers for EGFR pathway activation as predictor of outcome in metastatic colorectal cancer patients treated with or without cetuximab. Eur J Cancer 2010;46:1997-2009. [Crossref] [PubMed]
- Perkins G, Lièvre A, Ramacci C, et al. Additional value of EGFR downstream signaling phosphoprotein expression to KRAS status for response to anti-EGFR antibodies in colorectal cancer. Int J Cancer 2010;127:1321-31. [Crossref] [PubMed]
- Laurent-Puig P, Cayre A, Manceau G, et al. Analysis of PTEN, BRAF, and EGFR status in determining benefit from cetuximab therapy in wild-type KRAS metastatic colon cancer. J Clin Oncol 2009;27:5924-30. [Crossref] [PubMed]
- Van Cutsem E, Köhne CH, Láng I, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol 2011;29:2011-9. [Crossref] [PubMed]
- Karapetis CS, Jonker D, Daneshmand M, et al. PIK3CA, BRAF, and PTEN status and benefit from cetuximab in the treatment of advanced colorectal cancer--results from NCIC CTG/AGITG CO.17. Clin Cancer Res 2014;20:744-53. [Crossref] [PubMed]
- Yang SY, Cho MS, Kim NK. Difference between right-sided and left-sided colorectal cancers: from embryology to molecular subtype. Expert Rev Anticancer Ther 2018;18:351-8. [Crossref] [PubMed]
- Venook AP, Niedzwiecki D, Innocenti F, et al. Impact of primary (1º) tumor location on overall survival (OS) and progression-free survival (PFS) in patients (pts) with metastatic colorectal cancer (mCRC): Analysis of CALGB/SWOG 80405 (Alliance). J Clin Oncol 2016;34:3504. [Crossref]
- Arnold D, Lueza B, Douillard JY, et al. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann Oncol 2017;28:1713-29. [Crossref] [PubMed]
- Heinemann V, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab or bevacizumab for advanced colorectal cancer: final survival and per-protocol analysis of FIRE-3, a randomised clinical trial. Br J Cancer 2021;124:587-94. [Crossref] [PubMed]
- Tejpar S, Stintzing S, Ciardiello F, et al. Prognostic and Predictive Relevance of Primary Tumor Location in Patients With RAS Wild-Type Metastatic Colorectal Cancer: Retrospective Analyses of the CRYSTAL and FIRE-3 Trials. JAMA Oncol 2017;3:194-201. [Crossref] [PubMed]
- Van Emburgh BO, Sartore-Bianchi A, Di Nicolantonio F, et al. Acquired resistance to EGFR-targeted therapies in colorectal cancer. Mol Oncol 2014;8:1084-94. [Crossref] [PubMed]
- Diaz LA Jr, Williams RT, Wu J, et al. The molecular evolution of acquired resistance to targeted EGFR blockade in colorectal cancers. Nature 2012;486:537-40. [Crossref] [PubMed]
- Misale S, Yaeger R, Hobor S, et al. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature 2012;486:532-6. [Crossref] [PubMed]
- Sclafani F, Kim TY, Cunningham D, et al. A Randomized Phase II/III Study of Dalotuzumab in Combination With Cetuximab and Irinotecan in Chemorefractory, KRAS Wild-Type, Metastatic Colorectal Cancer. J Natl Cancer Inst 2015;107:djv258. [Crossref] [PubMed]
- Liska D, Chen CT, Bachleitner-Hofmann T, et al. HGF rescues colorectal cancer cells from EGFR inhibition via MET activation. Clin Cancer Res 2011;17:472-82. [Crossref] [PubMed]
- Montagut C, Dalmases A, Bellosillo B, et al. Identification of a mutation in the extracellular domain of the Epidermal Growth Factor Receptor conferring cetuximab resistance in colorectal cancer. Nat Med 2012;18:221-3. [Crossref] [PubMed]
- Esposito C, Rachiglio AM, La Porta ML, et al. The S492R EGFR ectodomain mutation is never detected in KRAS wild-type colorectal carcinoma before exposure to EGFR monoclonal antibodies. Cancer Biol Ther 2013;14:1143-6. [Crossref] [PubMed]
- Zhou J, Ji Q, Li Q. Resistance to anti-EGFR therapies in metastatic colorectal cancer: underlying mechanisms and reversal strategies. J Exp Clin Cancer Res 2021;40:328. [Crossref] [PubMed]
- Bouchahda M, Karaboué A, Saffroy R, et al. Acquired KRAS mutations during progression of colorectal cancer metastases: possible implications for therapy and prognosis. Cancer Chemother Pharmacol 2010;66:605-9. [Crossref] [PubMed]
- Misale S, Arena S, Lamba S, et al. Blockade of EGFR and MEK intercepts heterogeneous mechanisms of acquired resistance to anti-EGFR therapies in colorectal cancer. Sci Transl Med 2014;6:224ra26. [Crossref] [PubMed]
- Kim TW, Peeters M, Thomas A, et al. Impact of Emergent Circulating Tumor DNA RAS Mutation in Panitumumab-Treated Chemoresistant Metastatic Colorectal Cancer. Clin Cancer Res 2018;24:5602-9. [Crossref] [PubMed]
- Wheeler DL, Huang S, Kruser TJ, et al. Mechanisms of acquired resistance to cetuximab: role of HER (ErbB) family members. Oncogene 2008;27:3944-56. [Crossref] [PubMed]
- Scartozzi M, Mandolesi A, Giampieri R, et al. Insulin-like growth factor 1 expression correlates with clinical outcome in K-RAS wild type colorectal cancer patients treated with cetuximab and irinotecan. Int J Cancer 2010;127:1941-7. [Crossref] [PubMed]
- Scartozzi M, Giampieri R, Maccaroni E, et al. Analysis of HER-3, insulin growth factor-1, nuclear factor-kB and epidermal growth factor receptor gene copy number in the prediction of clinical outcome for K-RAS wild-type colorectal cancer patients receiving irinotecan-cetuximab. Ann Oncol 2012;23:1706-12. [Crossref] [PubMed]
- Gherardi E, Birchmeier W, Birchmeier C, et al. Targeting MET in cancer: rationale and progress. Nat Rev Cancer 2012;12:89-103. [Crossref] [PubMed]
- Troiani T, Martinelli E, Napolitano S, et al. Increased TGF-alpha as a mechanism of acquired resistance to the anti-EGFR inhibitor cetuximab through EGFR-MET interaction and activation of MET signaling in colon cancer cells. Clin Cancer Res 2013;19:6751-65. [Crossref] [PubMed]
- Ciardiello F, Bianco R, Caputo R, et al. Antitumor activity of ZD6474, a vascular endothelial growth factor receptor tyrosine kinase inhibitor, in human cancer cells with acquired resistance to antiepidermal growth factor receptor therapy. Clin Cancer Res 2004;10:784-93. [Crossref] [PubMed]
- Bianco R, Rosa R, Damiano V, et al. Vascular endothelial growth factor receptor-1 contributes to resistance to anti-epidermal growth factor receptor drugs in human cancer cells. Clin Cancer Res 2008;14:5069-80. [Crossref] [PubMed]
- Pilz LR, Manegold C, Schmid-Bindert G. Statistical considerations and endpoints for clinical lung cancer studies: Can progression free survival (PFS) substitute overall survival (OS) as a valid endpoint in clinical trials for advanced non-small-cell lung cancer? Transl Lung Cancer Res 2012;1:26-35. [PubMed]
- Dienstmann R, Serpico D, Rodon J, et al. Molecular profiling of patients with colorectal cancer and matched targeted therapy in phase I clinical trials. Mol Cancer Ther 2012;11:2062-71. [Crossref] [PubMed]
- Sartore-Bianchi A, Amatu A, Bonazzina E, et al. Pooled Analysis of Clinical Outcome of Patients with Chemorefractory Metastatic Colorectal Cancer Treated within Phase I/II Clinical Studies Based on Individual Biomarkers of Susceptibility: A Single-Institution Experience. Target Oncol 2017;12:525-33. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)