
Immune-related adverse events and prognosis in patients with upper gastrointestinal cancer treated with nivolumab
Introduction
Upper gastrointestinal tract (GIT) malignancies include cancers of the esophagus, gastroesophageal junction (GEJ), and stomach. Gastric and GEJ cancers are the fifth most common cancers and sixth leading cause of cancer-related deaths globally (1). A large proportion of patients with esophageal squamous cell carcinoma (ESCC) and gastric cancer (GC) is registered in East Asian countries, including Japan. Platinum-based doublet systemic chemotherapy is the standard treatment for advanced recurrent esophageal cancer and GC (2).
Recent advances in immune checkpoint inhibitors (ICIs) have improved treatment outcomes in several cancers, including upper GIT cancers. According to recent clinical trials, monoclonal antibodies targeting programmed cell death protein 1, such as nivolumab and pembrolizumab, have been approved for the treatment of unresectable, advanced, or recurrent ESCC and GC; compared with conventional cytotoxic chemotherapy, these drugs have been shown to increase survival (3,4). In Japan, nivolumab was approved as the third-line or later-line treatment option for unresectable, advanced, or recurrent GC in November 2017. Most recently, following the results of the CheckMate 649 and ATTRACTION-4 trials, nivolumab has been approved as the first-line treatment option for advanced or recurrent GC (4,5). Regarding the treatment of advanced or recurrent esophageal cancer, nivolumab is the approved second-line treatment for advanced or recurrent esophageal cancer since February 2020, following the results of the ATTRACTION-3 trial (6); meanwhile, pembrolizumab is the approved first-line treatment since November 2021, following the findings of the KEYNOTE-590 trial (7).
ICIs, such as nivolumab, cause imbalances in immunological tolerance, resulting in inflammatory side effects called immune-related adverse events (irAEs) (8). The development of irAEs is associated with survival in melanoma and non-small cell lung cancer, suggesting that early development of irAEs may predict better outcomes with ICIs and that prompt management of irAEs may prolong treatment duration and maximize the therapeutic effect of ICIs (9). Although several reports have shown that the occurrence of irAEs is associated with the prognosis of advanced GC treated with ICIs (10-13), there are few reports on advanced esophageal cancer and, therefore, there are insufficient data in this regard (2,8,10-13). This study aimed to investigate the prognostic impact of irAEs in patients with advanced or recurrent upper GIT cancer treated with nivolumab. We present the following article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-281/rc).
Methods
Patients
A total of 96 patients with advanced or recurrent upper GIT cancer, who were treated with nivolumab at Kumamoto University Hospital were enrolled in this study. In our hospital, nivolumab was authorized for the treatment of patients with GC and ESCC in November 2017 and July 2018, respectively. Patients were observed at 1–3-month intervals until death or October 31, 2021, whichever came first.
Procedure
Nivolumab was administered intravenously at a dose of 240 mg once every 2 weeks or 480 mg once every 4 weeks. Treatment efficacy was assessed using the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1. Treatment was administered until progressive disease was achieved or until the patient was deemed intolerant due to toxicity (14). Computed tomography was performed 2 to 3 months after treatment initiation or obvious disease progression; these images were used for the assessment of tumor activity according to the RECIST criteria. Adverse events were assessed from the treatment period to 1 month after the last dose or until the start of the next treatment according to the National Cancer Institute Common Terminology Criteria for Adverse Event, version 5.0 (15). At Kumamoto University Hospital, a cross-departmental immune-related adverse event control team (iREACT) was established to diagnose and to treat irAEs as early as possible. IrAEs are diagnosed mainly by the physician in charge; however, doctors, nurses, pharmacists, and other members of the team can also make a diagnosis. Because irAE is difficult to diagnose at an early stage and this is a retrospective study based on medical records, the type and definition of irAE was determined in this study by referring to previous papers on ICI treatments (4-7).
Statistical analyses
All statistical analyses were carried out using JMP version 14.2.0 software (SAS Institute, Cary, NC, USA). All P values were two-sided. We used fisher’s exact test and Chi-square test for categorical data and the t-test for quantitative data. Survival-time distribution in the survival analysis was assessed by the Kaplan-Meier method using log-rank tests. A Cox model was used for the univariate and multivariate analyses, as well as for the estimation of hazard ratios (HRs). A P value of <0.05 was considered as statistically significant.
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Medical Ethics Review Committee of Kumamoto University Hospital (No. 1909). The patients’ personal information was anonymized and informed consent was obtained from all participants.
Results
Patient characteristics
The characteristics of the participants are shown in Table 1. Ninety-six patients were included in this study: 35 (36.5%) with GC, include 10 (10.4%) with GEJ cancer, and 61 (63.5%) with esophageal cancer [squamous cell carcinoma (56, 58.3%), adenocarcinoma (1, 1.0%), and basaloid and carcinosarcoma (4, 4.2%)]. Nivolumab was used as second-line therapy in 35 (36.5%) patients, while third-line or later-line therapy was used for the remaining 61 (63.5%) patients. Second-line treatment was used mostly for esophageal cancer cases (31, 51.0%), while third-line therapy was used mostly for GC (28, 80%). Of all the patients, 41 (42.7%) experienced irAEs; irAE-related details are shown in Table 2. The commonest irAE was fatigue, followed by skin rashes and decreased appetite. Four patients had Grade 3 irAEs—severe disease. No life-threatening adverse events of Grade 4 or higher were observed. Patients with irAEs had a significantly better Eastern Cooperative Oncology Group performance status (ECOG PS) (P=0.01) and were administered nivolumab more than those without irAEs (P=0.003). IrAEs were unrelated to age, sex, tumor location, first treatment, tumor histology, number of organ metastases, site of metastasis, and therapy after nivolumab administration. The risk factors for irAEs included good ECOG PS (P=0.0003) and high albumin level (P=0.0077) (Figure 1). Paradoxically, irAEs were less likely to occur in patients with a poor general health or nutritional status.
Table 1
| Characteristic | All patients | irAE (−) | irAE (+) | P value |
|---|---|---|---|---|
| Total | 96 | 55 (57.3%) | 41 (42.7%) | |
| Age (years), mean ± SD | 64±10.8 | 66±12.0 | 62±9.1 | 0.82 |
| Sex, n (%) | 0.27 | |||
| Male | 72 (75.0) | 39 (70.9) | 33 (80.5) | |
| Female | 24 (25.0) | 16 (29.1) | 8 (19.5) | |
| ECOG PS, n (%) | 0.01 | |||
| ≤1 | 79 (82.3) | 40 (72.7) | 39 (95.1) | |
| ≥2 | 17 (17.7) | 15 (27.3) | 2 (4.9) | |
| Body mass index (kg/m2), mean ± SD | 19.2±2.9 | 18.8±2.9 | 19.7±3.0 | 0.15 |
| Tumor location, n (%) | 0.34 | |||
| Esophagus | 60 (62.5) | 31 (56.4) | 29 (70.7) | |
| Esophagogastric junction | 11 (11.5) | 7 (12.7) | 4 (9.8) | |
| Stomach | 25 (26.0) | 17 (30.9) | 8 (19.5) | |
| First treatment, n (%) | 0.86 | |||
| Surgery | 41 (42.7) | 24 (43.6) | 17 (41.5) | |
| Chemotherapy | 37 (38.5) | 20 (36.4) | 17 (41.5) | |
| Chemoradiation therapy | 18 (18.8) | 11 (20.0) | 7 (17.0) | |
| Histology, n (%) | 0.35 | |||
| Squamous cell carcinoma | 56 (58.3) | 29 (52.7) | 27 (65.9) | |
| Adenocarcinoma | 36 (37.5) | 24 (43.6) | 12 (29.3) | |
| Others | 4 (4.2) | 2 (3.7) | 2 (4.8) | |
| Number of organs with metastases, n (%) | 0.48 | |||
| ≤1 | 57 (59.4) | 31 (56.4) | 26 (63.4) | |
| ≥2 | 39 (40.6) | 24 (43.6) | 15 (36.6) | |
| Site of metastases, n (%) | ||||
| Lymph node | 51 (53.1) | 26 (47.3) | 25 (60.9) | 0.18 |
| Peritoneum/pleural | 26 (27.1) | 16 (29.1) | 10 (24.4) | 0.60 |
| Lung | 21 (21.9) | 14 (25.5) | 7 (17.1) | 0.32 |
| Liver | 24 (25.0) | 16 (29.1) | 8 (19.5) | 0.28 |
| Bone | 7 (7.3) | 5 (9.1) | 2 (4.9) | 0.43 |
| Therapy line, n (%) | 0.42 | |||
| 2nd | 35 (36.5) | 19 (34.6) | 16 (39.0) | |
| 3rd | 49 (51.0) | 31 (56.4) | 18 (43.9) | |
| ≥4th | 12 (12.5) | 5 (9.1) | 7 (17.1) | |
| Number of administrations, n (%) | 0.003 | |||
| ≤4 | 54 (56.3) | 38 (69.1) | 16 (39.0) | |
| ≥5 | 42 (43.7) | 17 (30.9) | 25 (61.0) | |
| After nivolumab therapy, n (%) | 0.35 | |||
| Yes | 28 (29.2) | 14 (25.5) | 14 (34.2) | |
| No | 68 (70.8) | 41 (74.5) | 27 (65.8) |
irAE, immune-related adverse event; ECOG PS, Eastern Cooperative Oncology Group performance status; SD, standard deviation.
Table 2
| Category | Total (n=96) (%) | Grade 1–2 (%) | Grade 3–4 (%) |
|---|---|---|---|
| Any | 41 (42.7) | 37 (38.5) | 4 (4.2) |
| Pneumonitis | 1 (1.0) | 1 (1.0) | 0 (0.0) |
| Endocrine | |||
| Thyroiditis/hypothyroidism | 4 (4.2) | 4 (4.2) | 0 (0.0) |
| Hypoadrenalism | 5 (5.2) | 3 (3.1) | 2 (2.1) |
| Hypopituitarism | 1 (1.0) | 1 (1.0) | 0 (0.0) |
| Type 1 diabetes | 1 (1.0) | 0 (0.0) | 1 (1.0) |
| Gastrointestinal | |||
| Diarrhea/colitis | 4 (4.2) | 3 (3.1) | 1 (1.0) |
| Dysgeusia | 2 (2.1) | 2 (2.1) | 0 (0.0) |
| Stomatitis | 3 (3.1) | 3 (3.1) | 0 (0.0) |
| Rash | 9 (9.4) | 9 (9.4) | 0 (0.0) |
| Fatigue | 14 (14.7) | 14 (14.7) | 0 (0.0) |
| Decreased appetite | 8 (8.4) | 8 (8.4) | 0 (0.0) |
| Renal dysfunction | 2 (2.1) | 2 (2.1) | 0 (0.0) |
| Liver dysfunction | 2 (2.1) | 2 (2.1) | 0 (0.0) |
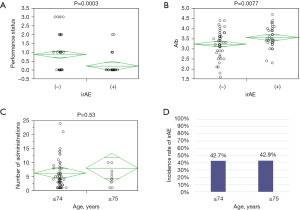
Of the 96 patients, 14 were aged above 75 years. Notably, the oldest patient was aged 89 years (range, 34–89 years). The frequency of irAEs and number of times nivolumab was administered were not different between the elderly (≥75) and non-elderly (≤74) groups. These results indicate that nivolumab can be administered safely to elderly patients in clinical practice (Figure 1).
Therapeutic effect
The best overall response (BOR) is shown in Table 3. Of all the patients, 11 (11.5%) and 20 (20.8%) achieved partial response and stable disease, respectively. None of the patients achieved complete response to nivolumab. The BOR rates were compared between patients with and without irAEs; this comparison excluded 4 (4.2%) patients with unevaluable tumor responses. Patients with irAEs (n=41) had a significantly better BOR than those without irAEs (n=55) (P=0.014). Overall, the response rate (RR) was 11.5%, and the disease control rate (DCR) was 34.4% (Table 3). The RR and DCR results were better in patients with irAEs.
Table 3
| Effectiveness evaluation | Classification | N=96 | irAE (−) | irAE (+) | P value |
|---|---|---|---|---|---|
| Partial response | 11 (11.5%) | 3 | 8 | 0.014 | |
| Stable disease | 20 (20.8%) | 9 | 11 | ||
| Progressive disease | 59 (+pseudo PD 2) | 41 (+pseudo PD 1) | 18 (+pseudo PD 1) | ||
| Not evaluable | 4 | 2 | 2 | ||
| First CT judgment | PR:SD:PD [pseudo PD]:NE | 11:20:59 [2]:4 | |||
| Response rate | CR + PR | 11 (11.5%) | 3 | 8 | 0.032 |
| Disease control rate | CR + PR + SD (pseudo PD) | 33 (34.4%) | 12 | 21 | 0.003 |
CT, computed tomography; PR, partial response; SD, stable disease; PD, progressive disease; CR, complete response; NE, not evaluable.
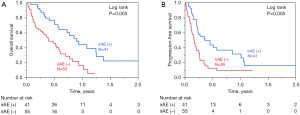
Relationship between irAEs and overall survival (OS) and progression free survival (PFS)
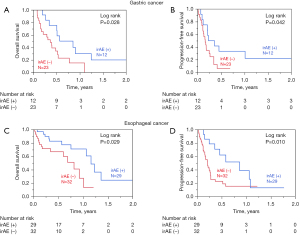
The Kaplan-Meier curves for OS and PFS of all the 96 patients according to the incidence of irAEs are shown in Figure 2. Patients with irAEs (n=41) had a significantly better OS than those without irAEs (n=55, log-rank P=0.003). Subgroup analyses revealed that patients with GC and irAEs (n=12) had a significantly better OS and PFS than those without irAEs (n=23; Figure 3A, OS: log-rank P=0.028; Figure 3B, PFS: log-rank P=0.042). In esophageal cancer, OS and PFS were significantly better in those with irAEs (n=29) (Figure 3C, OS: log-rank P=0.029; Figure 3D, PFS: log-rank P=0.010).
Prognostic factors and irAE occurrence
The univariate analysis revealed that female sex, adenocarcinoma, body mass index <18.5 kg/m2, ECOG PS score ≥2, and absence of irAEs were clinically important factors affecting the OS of patients with upper GIT cancer treated with nivolumab. In the multivariate analysis, male sex [HR =0.43, 95% confidence interval (CI) =0.23–0.79, P=0.007] and presence of an irAE (HR =0.47; 95% CI =0.26–0.88; P=0.018) were good prognostic factors. An ECOG PS score ≥2 (HR =4.51; 95% CI =2.08–9.73; P<0.001) was identified as an independent poor prognostic factor (Table 4).
Table 4
| Variables | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Age (≥70/<70 years) | 1.22 | 0.69–2.14 | 0.50 | – | – | – | |
| Sex (male/female) | 0.41 | 0.24–0.73 | <0.01 | 0.43 | 0.23–0.79 | 0.007 | |
| Histology (SCC/adeno) | 0.58 | 0.34–0.97 | 0.03 | 0.71 | 0.40–1.28 | 0.26 | |
| Number of meta (≥2/≤1) | 1.41 | 0.84–2.34 | 0.18 | – | – | – | |
| irAE (+/−) | 0.36 | 0.21–0.65 | <0.01 | 0.47 | 0.26–0.88 | 0.018 | |
| BMI (≥18.5/<18.5 kg/m2) | 0.44 | 0.26–0.77 | <0.01 | 0.56 | 0.30–1.05 | 0.71 | |
| ECOG PS (≥2/≤1) | 5.68 | 2.95–10.9 | <0.01 | 4.51 | 2.08–9.73 | <0.001 | |
SCC/adeno, squamous cell carcinoma/adenocarcinoma; irAE, immune-related adverse event; BMI, body mass index; ECOG PS, Eastern Cooperative Oncology Group performance status.
Discussion
ICIs have become an extremely important treatment option for upper GIT cancers. Moreover, the management of associated irAEs has become clinically important. Although it has been reported that patients with irAEs have a better prognosis in various carcinomas (13-17), for example non-small cell lung cancer and melanoma, the relationship between irAEs and treatment response in upper GIT cancer remains unclear. In this study of 96 upper GIT cancer cases, we found that the prognosis of both esophageal cancer and GC was better in patients with irAEs than in those without. We also showed that irAEs are less likely to occur in patients with a poor general health or nutritional status and that nivolumab can be safely administered to patients aged above 75 years.
Some studies have focused on the relationship between irAEs and clinical outcomes in patients with upper GIT cancer. In these studies, patients with GC who developed irAEs had a good prognosis. However, the sample size in most of these studies was less than 70. Satoh et al. in the ATTRACTION-1/ONO-4538-07 trial reported that patients with selected adverse events tended to have a better OS than those without (13). However, the relationship between irAEs and prognosis in clinical practice, rather than in clinical trials, has not been clarified.
In this study, we found only four cases of severe irAEs of Grade 3 or higher. The number of severe irAEs was significantly smaller than that in previous reports, such as ATTRACTION-2 and ATTRACTION-3 (6,18). One reason for this is that the iREACT in our hospital performed their functions well; the team shares information on irAEs not only with physicians in each department, including the attending physician, but also with other medical staff members, such as nurses and pharmacists—this enables early detection, reliable diagnosis, and treatment. Esophageal cancer cases had a higher incidence of irAEs (47.5% vs. 34.2%); however, there was no significant difference between this frequency and that in GC cases. The impact of second- and third-line treatments in our study patients was significant. The difference between use in second-line treatment and use in third-line treatment may have affected the length of use and the number of doses. It should be noted that nivolumab is used as a second-line treatment for esophageal cancer but as a third-line for GC. The same effect is expected in squamous cell carcinoma and adenocarcinoma. In addition, the effects of pre-treatment and administration of post-treatment are also considered as having a significant impact; future studies and analyses should take these factors into consideration. Furthermore, it is necessary to pay attention to the timing of the onset of irAEs and to clarify the causal relationship between irAEs and prognosis. It is necessary to examine whether the prognosis is good because irAEs occurred or whether irAEs occurred because nivolumab was highly effective and could be used for a long time.
The mechanism by which irAEs are associated with treatment response and prognosis remains unclear. According to previous reports, vitiligo and rash in melanoma and thyroid dysfunction and multiple organ involvement in lung cancer have been reported to be associated with improved prognosis (19-25). The main mechanism of an irAE is thought to be the destruction of autologous cells and tissues by autoantibodies and accidental activation of autoantigen-specific lymphocytes, which are produced and remain in the body without being removed, following administration of ICIs. However, it is difficult to describe irAEs in terms of a simple pathway because of their variation with cancer types and symptoms (26).
IrAEs were more likely to occur in patients with good nutritional and general conditions, such as high serum albumin levels and a good ECOG PS. In the multivariate analysis, a good ECOG PS was also a prognostic factor. Therefore, early administration of nivolumab with appropriate management of irAEs and maintenance of the systemic status as much as possible is important for achieving therapeutic efficacy. This also suggests that irAEs may be less likely to occur in patients with a poor general health status and that an opportunity exists to administer nivolumab to elderly patients. Ten patients aged 75 years or older (10.4%; range 75–89 years) were also included in this study, and there was no increase in the frequency of irAEs or decrease in the number of administrations due to older age. Therefore, nivolumab could be safely administered to elderly patients.
This study had some limitations. First, this was a retrospective, single-center investigation with a small number of patients. Second, irAEs that were not explicitly documented in medical records were not included in the study. Further studies with larger cohorts are needed to confirm the association between irAE development and nivolumab efficacy.
In conclusion, the incidence of irAEs is a prognostic factor for upper gastrointestinal cancers treated with nivolumab, nivolumab can be administered to patients aged 75 years and older, and appropriate management of irAEs is necessary to maximize therapeutic efficacy.
Acknowledgments
The authors thank all the patients and medical staff at the institutions that contributed to this study.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-281/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-281/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-281/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Medical Ethics Review Committee of Kumamoto University hospital (No. 1909). The patients’ personal information was anonymized and informed consent was obtained from all participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Booka E, Kikuchi H, Haneda R, et al. Impact of Immune-related Adverse Events on Nivolumab Efficacy in Patients With Upper Gastrointestinal Cancer. In Vivo 2021;35:2321-6. [Crossref] [PubMed]
- Smyth EC, Gambardella V, Cervantes A, et al. Checkpoint inhibitors for gastroesophageal cancers: dissecting heterogeneity to better understand their role in first-line and adjuvant therapy. Ann Oncol 2021;32:590-9. [Crossref] [PubMed]
- Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet 2021;398:27-40. [Crossref] [PubMed]
- Boku N, Ryu MH, Kato K, et al. Safety and efficacy of nivolumab in combination with S-1/capecitabine plus oxaliplatin in patients with previously untreated, unresectable, advanced, or recurrent gastric/gastroesophageal junction cancer: interim results of a randomized, phase II trial (ATTRACTION-4). Ann Oncol 2019;30:250-8. [Crossref] [PubMed]
- Takahashi M, Kato K, Okada M, et al. Nivolumab versus chemotherapy in Japanese patients with advanced esophageal squamous cell carcinoma: a subgroup analysis of a multicenter, randomized, open-label, phase 3 trial (ATTRACTION-3). Esophagus 2021;18:90-9. [Crossref] [PubMed]
- Sun JM, Shen L, Shah MA, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet 2021;398:759-71. Erratum in: Lancet 2021;398:1874. [Crossref] [PubMed]
- Masuda K, Shoji H, Nagashima K, et al. Correlation between immune-related adverse events and prognosis in patients with gastric cancer treated with nivolumab. BMC Cancer 2019;19:974. [Crossref] [PubMed]
- Haratani K, Hayashi H, Chiba Y, et al. Association of Immune-Related Adverse Events With Nivolumab Efficacy in Non-Small-Cell Lung Cancer. JAMA Oncol 2018;4:374-8. [Crossref] [PubMed]
- Kono Y, Choda Y, Nakagawa M, et al. Association Between Immune-Related Adverse Events and the Prognosis of Patients with Advanced Gastric Cancer Treated with Nivolumab. Target Oncol 2021;16:237-48. [Crossref] [PubMed]
- Ando T, Ueda A, Ogawa K, et al. Prognosis of Immune-related Adverse Events in Patients With Advanced Gastric Cancer Treated With Nivolumab or Pembrolizumab: A Multicenter Retrospective Analysis. In Vivo 2021;35:475-82. [Crossref] [PubMed]
- Namikawa T, Yokota K, Tanioka N, et al. Systemic inflammatory response and nutritional biomarkers as predictors of nivolumab efficacy for gastric cancer. Surg Today 2020;50:1486-95. [Crossref] [PubMed]
- Satoh T, Kato K, Ura T, et al. Five-year follow-up of nivolumab treatment in Japanese patients with esophageal squamous-cell carcinoma (ATTRACTION-1/ONO-4538-07). Esophagus 2021;18:835-43. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Common terminology criteria for adverse events (CTCAE) Version 5.0, 2017. Available online: https://ctep.cancer.gov/ProtocolDevelopment/electronic_applications/ctc.htm#ctc_50 [Last accessed on May 20, 2021].
- Indini A, Di Guardo L, Cimminiello C, et al. Immune-related adverse events correlate with improved survival in patients undergoing anti-PD1 immunotherapy for metastatic melanoma. J Cancer Res Clin Oncol 2019;145:511-21. [Crossref] [PubMed]
- Maillet D, Corbaux P, Stelmes JJ, et al. Association between immune-related adverse events and long-term survival outcomes in patients treated with immune checkpoint inhibitors. Eur J Cancer 2020;132:61-70. [Crossref] [PubMed]
- Kang YK, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;390:2461-71. [Crossref] [PubMed]
- Owen DH, Wei L, Bertino EM, et al. Incidence, Risk Factors, and Effect on Survival of Immune-related Adverse Events in Patients With Non-Small-cell Lung Cancer. Clin Lung Cancer 2018;19:e893-e900. [Crossref] [PubMed]
- Suo A, Chan Y, Beaulieu C, et al. Anti-PD1-Induced Immune-Related Adverse Events and Survival Outcomes in Advanced Melanoma. Oncologist 2020;25:438-46. [Crossref] [PubMed]
- Verzoni E, Cartenì G, Cortesi E, et al. Real-world efficacy and safety of nivolumab in previously-treated metastatic renal cell carcinoma, and association between immune-related adverse events and survival: the Italian expanded access program. J Immunother Cancer 2019;7:99. [Crossref] [PubMed]
- Nakamura Y, Tanaka R, Asami Y, et al. Correlation between vitiligo occurrence and clinical benefit in advanced melanoma patients treated with nivolumab: A multi-institutional retrospective study. J Dermatol 2017;44:117-22. [Crossref] [PubMed]
- Hua C, Boussemart L, Mateus C, et al. Association of Vitiligo With Tumor Response in Patients With Metastatic Melanoma Treated With Pembrolizumab. JAMA Dermatol 2016;152:45-51. [Crossref] [PubMed]
- Osorio JC, Ni A, Chaft JE, et al. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann Oncol 2017;28:583-9. [Crossref] [PubMed]
- Shankar B, Zhang J, Naqash AR, et al. Multisystem Immune-Related Adverse Events Associated With Immune Checkpoint Inhibitors for Treatment of Non-Small Cell Lung Cancer. JAMA Oncol 2020;6:1952-6. [Crossref] [PubMed]
- Kubo T, Hirohashi Y, Tsukahara T, et al. Immunopathological basis of immune-related adverse events induced by immune checkpoint blockade therapy. Immunol Med 2022;45:108-18. [Crossref] [PubMed]


