
Rectal gastrointestinal stromal tumor with metachronous liver metastasis demonstrated no relapse after multidisciplinary team discussion and comprehensive treatment: a case report
Introduction
Gastrointestinal stromal tumors (GISTs) originate from the interstitial cells of Cajal and are the most common mesenchymal tumors of the gastrointestinal tract (1). The most common location of GIST is the stomach (accounting for about 60% of all GIST cases), followed by the small intestine (accounting for about 35%), and these tumors are less commonly observed in the colon-rectum (2). GIST metastasize easily. The liver is the most common site for GIST metastasis, and accounts for 55–72% of all distant metastasis cases (3). It has been reported that about 17% of GIST patients have liver metastasis at 1st presentation, and >70% of patients develop liver metastasis even after radical resection (4).
Surgery was once the only effective treatment for GIST; however, the postoperative recurrence and metastasis rates remained relatively high even after the complete resection of the high-risk GIST. The advent of tyrosine kinase inhibitors (TKIs) and development of molecular testing in recent decades has dramatically changed the treatment paradigm for GIST. Today, radical surgical resection combined with targeted therapy has become a standard treatment modality for GIST (5). From the Consensus on the diagnosis and treatment of gastrointestinal stromal tumors in China (2008 edition) (6) to the Expert consensus on the diagnosis and treatment of gastrointestinal stromal tumors in China (2011 edition) (7), to the Consensus on the diagnosis and treatment of gastrointestinal stromal tumors in China (2013 edition) (8), to the Chinese consensus guidelines for diagnosis and management of gastrointestinal stromal tumor (2017 edition) (9), and to the latest the Chinese expert consensus on whole-process management of gastrointestinal stromal tumor (2020 edition) (10), it can be seen that the development of the treatment concept of GIST in China has changed from the old traditional management model to the whole-process management, which indicates that multidisciplinary team (MDT) in the management of GIST is an inevitable product of the development of the era of precision treatment. In recent years, MDT discussion has been introduced to the diagnosis and treatment of GIST to provide individualized multidisciplinary treatments to GIST patients.
The incidence of rectal GIST is low, and metachronous liver metastases (MLMs) from rectal GIST are even rarer. At our center (the Department of Colorectal Surgery, The Third Affiliated Hospital of Kunming Medical University), a patient with MLMs from rectal GIST received multi-disciplinary management and standardized diagnosis and treatment using the MDT approach. No evidence of disease (NED) activity was achieved, and to date, the patient has survived for >7 years. The case presentation is as below. We present the following article in accordance with the CARE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-990/rc).
Case presentation
A 53-year-old male patient was admitted to Yunnan Cancer Hospital on October 9, 2014. He was noted to have a rectal mass found by colonoscopy over a month prior to presenting. His baseline data were as follows: body mass index: 23.2 kg/m2; body surface area: 1.72 m2; Eastern Cooperative Oncology Group score: 1; Nutrition Risk Screening score: 1 point; and Ability of Daily Living Scale level: 1. Thoracic and abdominal physical examinations revealed no obvious abnormalities. During the digital anal examination in the knee-chest position, a raised and hard mass was observed on the right posterior wall of the rectum, 4 cm away from the anal verge. The mass was about 4.0 cm × 3.0 cm in size, and had a smooth surface. There was no tenderness, and the rectal mucosa was smooth. No blood stain was observed on the finger cuff.
Auxiliary examinations were undertaken after admission. During the whole treatment process, we provided the images of pelvic magnetic resonance imagining (MRI) and transrectal ultrasound (TRUS) response to targeted therapy with imatinib, as well as surgical specimens of rectal GIST (see Figure 1). An electronic colonoscopy on October 12, 2014 revealed that the raised mucosal mass was posterior to the rectum after entering the endoscope by 3 cm, and had a generally clear boundary. Pelvic MRI on October 14, 2014 (see Figure 1A,1B, upper) showed that the mass behind the rectum had roughly clear borders. It pushed against and adhered to the posterior wall of the rectum, causing the rectum to move to the left and anterior sides. In addition, it adhered locally to the adjacent levator ani muscle. The tumor was about 4.5 cm × 4.0 cm × 4.2 cm in size, and it was obviously unevenly enhanced after contrast enhancement. The possibility of a GIST was considered.
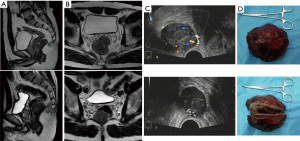
A TRUS on October 19, 2014 (see Figure 1C, upper) showed a solid space-occupying lesion posterior to the lower rectal segment 30 mm from the anus; the border between the mass and the rectum was unclear, and the bowel was compressed; the tumor was oval-shaped and about 53 mm × 48 mm × 42 mm in size, with clear borders. The possibility of a GIST was considered. An ultrasound-guided puncture of the right posterior rectal mass was performed, and the pathological examination showed spindle cell lesions. The immunohistochemical findings were as follows: CD117 (+), DOG-1 (+), CD34 (+), SMA (−), S-100 (−), SDHB (+), Ki-67 (+, about 1%), Vim (+), and CK (−) (mitotic rate was not performed). NEXT generation sequencing showed the C-kit exon 9 mutation.
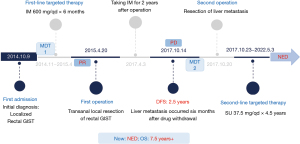
A diagnosis of a rectal GIST (C-kit exon 9-mutated) was made. A MDT discussion was held by departments of colorectal surgery, imaging, pathology and oncology, the following main opinions were expressed: For a large, localized GIST, neoadjuvant targeted therapy may be applied 1st to help shrink the tumor, reduce the surgical risk, increase the chance of radical resection, and protect the structures and functions of important organs and tissues such as anal sphincter, otherwise the standard surgery would have been abdominal perineal resection with permanent colostomy. Based on the body surface area and tolerance of Chinese patients and given the C-kit exon 9-mutated type in the current case, targeted therapy with oral imatinib (600 mg po qd) might be applied, and surgery could be performed after achieving the optimal therapeutic response (usually after 6–12 months). The treatment goal was “potentially curative”. Thus, the patient received oral imatinib (600 mg/qd) for 6 months from November 2014 to April 2015.
Subsequently, the patient underwent follow-up assessments. Pelvic MRI on April 14, 2015 (see Figure 1A,1B, lower) showed that after targeted therapy for the rectal GIST, the mass behind the rectum had roughly clear borders; it pushed against the posterior wall of the rectum, causing the rectum to move to the left and anterior sides. In addition, it adhered locally to the adjacent levator ani muscle. The tumor was about 3.5 cm × 2.8 cm × 3.2 cm in size, and it had obviously shrunk, and had obviously weakened enhancement. A TRUS on April 17, 2015 (see Figure 1C, lower) showed a solid space-occupying lesion posterior to the lower rectal segment 30 mm from the anus; the border between the mass and the rectum was unclear, and the bowel was compressed; the tumor was about 36 mm × 32 mm × 30 mm in size, which was remarkably smaller than before. According to the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 (11), the treatment response was assessed as partial response (PR).
A transanorectal local resection of GIST was performed on April 20, 2015 (see Figure 1D). The postoperative pathologic diagnosis after rectal GIST treatment revealed a spindle cell tumor. Immunohistochemical staining showed CD34 (vascular +), CD117 (+), DOG-1 (+), SDHB (+), Ki-67 (−), Vim (+), CK (−), and mitotic counts (1/50 HPF). NEXT generation sequencing showed the C-kit exon 9 insertion (Y503-F504 INS AY). The oral administration of imatinib (600 mg/qd) continued after discharge. No local recurrence or distant metastasis was observed during the regular follow-up visits. Imatinib treatment was stopped by the patient himself 2 years later because of the economy and some side effects.
A follow-up visit was arranged 2.5 years after the operation. The abdominal and pelvic CT on October 14, 2017 showed a hypodense mass in the lower segment of the right posterior lobe of the liver, with clear boundaries, that was about 5.4 cm × 5.2 cm in size. After enhancement, the tumor showed inhomogeneous enhancement in the arterial phase, continuous enhancement in the portal venous phase, and decreased enhancement in the delayed phase. The mass was considered malignant. Abdominal MRI on October 15, 2017 (see Figure 2A) revealed a mass with abnormal signals in segment VI of the liver. The lesion was about 5.6 cm × 5.4 cm × 5.7 cm in size, with a smooth border. In addition, it pushed against the right kidney. A diagnosis of liver metastasis was considered.
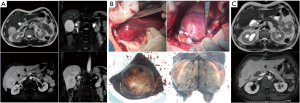
After the MDT discussion by departments of colorectal surgery, hepatobiliary surgery, imaging, pathology and oncology, the following main opinions were expressed: The lesion was a liver metastasis from GIST; the tumor was slightly large in size but had not invaded the important blood vessels and bile ducts in the liver. Radical resection was feasible, and the surgical risk was low, as the operation would not seriously affect the functions of related organs. The treatment goal was “potentially curative”. A resection of the liver metastasis and partial hepatectomy were performed on October 20, 2017 (see Figure 2B). After the operation, next generation sequencing showed the C-kit exon 9 mutation (A502-Y503lnsAY). Oral sunitinib (37.5 mg/d) was prescribed after surgery. The patient has been treated with sunitinib for 4.5 years since October 23, 2017, and no recurrence or metastasis has been observed during the re-examinations (see Figure 2C). Currently, the patient has a status of NED activity. A flow chart of the treatment process is shown in Figure 3.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013) (12). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
The incidence of GIST is about 1–2/100,000. Most GIST patients have the c-kit or platelet-derived growth factor receptor alpha gene mutation, but a smaller proportion of cases are driven by BRAF, SDH, and/or other gene mutations (13,14). GIST display complex biological behaviors, and range from benign to markedly malignant. The incidence of rectal GIST (which accounts for about 4–6% of all GIST) is lower than those of stomach and small bowel GIST, and most of rectal GIST are located in the middle and low rectum (15). The onset of rectal GIST is insidious and lacks specificity, which is related to the size, location, and invasiveness of the tumor. Its clinical manifestations mainly include changes in defecation habits, and bloody stool may be observed in a small number of patients. The lesions are often found incidentally during health check-ups. The results of routine pathological and immunohistochemical examinations are valuable in the diagnosis and differential diagnosis of rectal GIST. The tumor cells that can be observed under a light microscope are mainly spindle cells or epithelial cells. Immunohistochemistry often reveals positive expressions of CD117 and DOG-1. Endoscopy, endoscopic ultrasonography (EUS), CT, MRI, and other imaging modalities are the main examinations for rectal GIST. MRI and EUS have good sensitivity and specificity, and the combined application of these techniques can improve the accuracy of rectal GIST diagnosis.
Surgery was once the only effective treatment for rectal GIST. During the surgical resection of rectal GIST, care should be taken to maintain the integrity of the pseudo-capsule of the tumor, to avoid tumor rupture, and to achieve the en-bloc resection of the tumor. Rectal GIST are mostly located in the middle 3rd and lower 3rd of the rectum. Due to the special anatomical locations of rectal GIST, the complete resection of the tumor and the preservation of anal function must be carefully balanced. Research has shown that, under the premise of ensuring complete resection, local excision has better short-term efficacy than radical excision, and comparable long-term efficacy (16). Surgeons should be particularly cautious when choosing a surgical procedure. The optimal surgical method can be affected by a variety of factors, including the size, location, and local invasion of the tumor. Under the premise of achieving complete resection, the damage to the surrounding tissue must be minimized and the normal functions of the rectum and anal canal should be preserved. Since lymph node metastases from rectal GIST are extremely rare, routine lymph node dissection is not recommended. However, the postoperative recurrence and metastasis rates remain high even after the complete resection of the rectal GIST.
As the pathogenic mechanisms of GIST have been well elucidated, the TKI imatinib mesylate, the 1st TKI-targeted drug imatinib mesylate has dramatically improved the clinical outcomes of GIST patients and has become the preferred treatment for locally advanced rectal GIST (5). It is currently believed that preoperative treatment can be performed for rectal GIST for which it is difficult to achieve R0 resection or for those with a high surgical risk, a large size, and that require combined organ resection. The main objectives of preoperative neoadjuvant therapy are to shrink the tumor, reduce the extent of surgery, lower the risk of surgery, increase the chance of radical resection, protect the structures and functions of important organs, such as the anal sphincter, and ultimately improve the prognosis of patients. Regular re-examinations should be arranged once the imatinib treatment is initiated. Imaging examinations are valuable in assessing any changes in condition and therapeutic responses. When the treatment response is maximized or the disease is in a stable state, surgical resection should be performed as soon as possible. The excessive prolongation of preoperative treatment may lead to secondary drug resistance. The recommended preoperative treatment duration is 6 to 12 months.
The volume of rectal GIST should be reduced after neoadjuvant treatment, making it possible to perform local excision via the anal, sacrococcygeal, and vaginal approaches. In addition to achieving R0 resection, these procedures are less invasive and do not cause major damage to the anatomical structures and organ functions (17). In recent years, the important roles of MDT discussion in developing targeted therapy protocols, evaluating surgical indications, formulating safe and feasible surgical plans, and arranging postoperative follow-up visits have increasingly been recognized. As rectal GIST are special lesions, MDT discussion is highly valuable in the diagnosis and treatment of locally advanced rectal GIST.
In the present case, the patient was initially diagnosed with a locally advanced rectal GIST (C-kit exon 9-mutated). After the MDT discussion, the tumor shrank after neoadjuvant therapy with imatinib (600 mg), and the patient then underwent the transanorectal local resection of the GIST. R0 resection was achieved, and the anal sphincter function was preserved. After the operation, oral imatinib (600 mg) treatment was continued for 2 years. No local recurrence or distant metastasis was observed in the re-examinations, and a satisfactory therapeutic response and quality of life were achieved.
Research has shown that postoperative adjuvant therapy can prolong the time to recurrence and metastasis and improve the prognosis of patients with rectal GIST (18). The duration of adjuvant therapy can be selected according to the GIST risks; adjuvant therapy may last 2 years for intermediate-risk patients and at least 3 years for high-risk patients, and can be further prolonged for patients with tumor rupture (19). In the present case, the patient himself stopped taking imatinib 2 years after the surgery, and only 6 months after the discontinuation, his liver was found to have metastatic lesions, which might be explained by the insufficient duration of the postoperative targeted therapy.
A study has shown that in-situ local recurrence is common in rectal GIST, but metastasis to the liver, lungs, peritoneum, and bones can also occur (15). The liver is the most common site for the distant metastasis of GIST, and 55%~72% of relapsed patients have liver metastasis. Roberts et al. (20) found that the incidence of liver metastases from GIST was 15.9%, and only 10–20% of patients achieved complete surgical resection of the lesions; in addition, the recurrence rate after surgical resection of liver metastases reached 77%. In another study, the 2- and 5-year disease-free survival (DFS) rates of patients with GIST liver metastases after surgery were 30% and 20%, respectively (21). Thus, surgical resection remains the 1st choice for the treatment of GIST liver metastases, but the high postoperative recurrence rate makes an active surveillance strategy after surgery important to detect and treat metastatic disease.
The surgical treatment of simple liver metastases after surgery for GIST may be performed in patients with a good general condition, a Child-Pugh of A or B, and no diffuse extrahepatic metastasis. Radical liver resection may be feasible if: (I) the lesion is solitary and invades <30% of the liver tissue; or (II) the multiple lesions (<3) are localized within one lobe or segment. For GIST liver metastasis, the surgical methods include resection of the liver metastasis, partial hepatectomy, lobectomy, and even subtotal hepatectomy. In principle, the surgery needs to minimize the resection of normal liver tissue while completely removing the liver metastasis to prevent the occurrence of liver failure. Both the no-touch isolation technique and prevention of tumor rupture are key steps in preventing the hematogenous dissemination of tumors.
In the present case, the patient developed liver metastasis in segment VI 6 months after drug discontinuation. According to the MDT discussion, for this rectal GIST patient, whose tumor metastasized after adjuvant therapy and who discontinued imatinib treatment, a R0 resection would be achievable with little surgical risk and without seriously affecting the functions of the related organs if the metastatic lesion was solitary. Thus, the metastatic lesion was directly resected, and targeted therapy administered according to intraoperative findings and postoperative next generation sequencing results. It can be seen from the above that for GIST patients with liver metastasis, MDT mode can not only evaluate whether the metastatic lesions can be removed, so as to ensure high-quality diagnosis and treatment suggestions and the best treatment plan, avoid excessive diagnosis and treatment, mis-diagnosis and mis-treatment, and maximize the benefits of patients, but also can shorten the visit time of patients and significantly reduce the treatment costs of patients.
Zhu et al. (22) found that the progression-free survival (PFS) and overall survival (OS) of patients with simple liver metastases were significantly better than those of patients with abdominal and pelvic implantation metastases. Additionally, liver metastasis was not a predictor of poor prognosis for advanced GIST; indeed, patients with only liver lesions had the best prognosis. For patients with GIST liver metastases, surgery combined with adjuvant TKI therapy is currently the most effective treatment. A study of 49 patients with GIST liver metastases by Brudvik et al. (23) showed that the 5-year recurrence-free survival rate of patients treated with surgery plus TKI (47.1%) was significantly higher than that of surgical resection alone (9.5%) (P=0.013). Additionally, Nunobe et al. (24) demonstrated that the recurrence rate after initial hepatectomy was as high as 94%; a surgical cure was difficult due to the high frequency of repeat metastases. Thus, adjuvant targeted therapy should be used in the treatment of metastatic GIST. Turley et al. (25) retrospectively analyzed the prognosis of 39 patients undergoing hepatectomy for GIST liver metastases and confirmed that TKI treatment after surgery increased the survival rate [hazard ratio (HR) =0.04, P=0.006]. Xiao et al. (26) also confirmed that TKI adjuvant therapy following liver resection achieved a 5-year survival rate of up to 85.7%.
Synchronous liver metastases and MLMs do not have a significant effect on the prognosis of GIST patients. Shi et al. (27) examined 41 patients with synchronous liver metastases and 103 patients with MLMs from GIST, and found no significant difference between the two groups in terms of OS (P=0.734). Xiao et al. (26) conducted a single-center retrospective study with 51 patients with synchronous liver metastases and 51 patients with MLMs, and showed that both the extent of disease and the phase of metastasis did not affect either PFS (P=0.140) or OS (P=0.239). Seesing et al. (28) examined 20 patients with synchronous liver metastases and 27 patients with MLMs and found no significant difference in the median OS or 5-year survival rates between the two groups.
In relation to the postoperative treatment of GIST liver metastases, it is currently believed that patients at intermediate or high risk should continue to receive TKI therapy after surgery, which will lead to longer DFS and OS (29). If R0 resection of the lesion is achieved, TKI therapy can be maintained for at least 3 years; if R0 resection cannot be achieved, long-term TKI use is required. Due to the potential toxicity of drug therapy, the question of whether to continue TKI treatment even after R0 resection and the choice of TKI preparations remain controversial. At present, there is still no consensus as to the duration for TKI use after the radical resection of simple liver metastases from GIST.
The Stop GIST Trial (NCT02924714), an ongoing multicenter prospective study, is evaluating whether targeted therapy can be discontinued after the complete resection of GIST oligometastases. Turley et al. (25) analyzed the clinical data of 39 patients who underwent hepatic resection. Among them, 24 received imatinib after surgery, with a median treatment duration of 44 days, and 6 patients received sunitinib after surgery due to imatinib resistance, with a median therapy duration of 409 days. It was found that the length of postoperative TKI treatment was positively correlated with the improvement of patient survival (HR =0.04, P=0.006). A multicenter prospective clinical study conducted by Zhang et al. (30) aimed to compare the survival outcomes of patients with metastatic GIST who experienced tumor unifocal or multifocal progression after imatinib treatment and were then treated with different postoperative medications. Of the 97 patients enrolled in the study, 56 continued imatinib therapy after surgery (with the dose maintained at 400 mg/d in 43 patients and escalated to 600 mg/d in 13 patients) and 41 patients were switched to sunitinib treatment directly after surgery. The OS of the sunitinib and imatinib groups did not differ significantly (37.0 vs. 33.0 months, P=0.794), but the PFS of the sunitinib group was significantly longer than that of the imatinib group (30 vs. 12 months, P=0.009). The authors concluded that the PFS of patients with metastatic GIST could be prolonged by using to sunitinib after the operation after the progress of IM treatment. Similarly, in the current case, the patient had previously received imatinib treatment and underwent surgical resection for liver metastasis, and was then directly switched to sunitinib treatment for his postoperative therapy.
The pharmacokinetics of targeted drugs vary greatly among individuals, and some patients may develop drug resistance or disease progression due to low drug exposure (31). Therapeutic drug monitoring (TDM) is a medical practice that aims to maximize rational drug use. By individualizing drug treatment, it can guide tailored drug use by monitoring blood drug concentrations. TDM may be required in GIST patients taking imatinib and sunitinib to improve drug efficacy and reduce adverse reactions. However, limited by the conditions of our center, the patient in our current report did not receive a blood drug concentration test after receiving TKI treatment, and thus it was impossible to assess whether the therapeutic dose was reached in the patient. This was also a treatment deficiency in this case, and a limitation of the current analysis.
This rectal GIST harbored a C-kit exon 9 mutation. Sunitinib, which the patient was directly switched to after liver metastases, is a highly sensitive drug and has been administered to this patient for 4.5 years. Genetic testing plays an important role in drug selection and dosing.
Conclusions
Rectal GIST has a low incidence and is clinically non-specific, and the incidence of MCM after surgery for rectal GIST is even rarer. Neoadjuvant targeted therapy combined with surgery has become the standard treatment for locally advanced rectal GIST, and radical surgery plus targeted therapy is also the optimal treatment strategy for patients with GIST liver metastases. For patients with GIST liver metastasis, patient-centered precise diagnosis (based on both pathology and imaging techniques), precision therapy (a combination of surgery with targeted therapy), and MDT discussions are essential in developing optimal treatment goals and strategies for individual patients (32). The survival and quality of life of patients may also be improved through whole-process management and regular follow-up.
Questions to be further discussed and considered
Question 1: How long should the patient continue to take sunitinib?
Emerson Y. Chen: No more than 5 years.
Olugbenga Olowokure: Sunitinib no standard but 3–5 years reasonable and if NED, would start active surveillance with 3 monthly visit and 6 monthly scans.
Alessandro Mazzocca: Adjuvant therapy should be continued for at least 3 years after metastasectomy. The patient continued therapy with Sunitinib more than four years without evidence of disease progression. In this case I agree with decision to stop sunitinib and start follow-up, even if this should be considered a metastatic disease.
Question 2: How should the patient be treated after disease progression?
Emerson Y. Chen: Re-biopsy for mutation testing. Choices are imatinib 800 mg/d or sunitinib restart, and later on regorafenib.
Olugbenga Olowokure: Depends on extent of Progression and size and site of metastatic lesion as this patient has already received imatinib and sunitinib if still a surgical candidate would surgically resect obtain mutation testing ensure no new surprising mutation and then start a new agent like regorafenib although one could consider a clinical trial or going back on imatinib at a higher dose if previously well tolerated.
Alessandro Mazzocca: In c-KIT exon 9-mutated metastatic GIST, Imatinib at the dose of 800 mg can be more active than standard dose (400 mg). In case of Imatinib failure, Sunitinib is the right choice. In young patients, as this one, the standard dose (50 mg 4 on/2 off), should be considered.
Question 3: How should the R0 resection and surgical timing of the liver metastases of the GIST be evaluated?
Emerson Y. Chen: With any future metastases, generally one would start with systemic treatment first before surgery. Exceptions can be made if the surgery is straightforward.
Olugbenga Olowokure: For future liver metastasis as part of surveillance to catch this early would follow patient with MRI or CT scans every 6 months, if develops liver mets after review at multidisciplinary tumor board if surgeons feel strongly that they can resect depending on the size and site of the lesion, would likely first resect prior to additional TKI but TKI can also be considered initially. The final decision will be dependent on joint decision after review at MDT.
Alessandro Mazzocca: Abdominal MRI could be the right assessment to evaluate R0 resection. Surgical timing of the liver metastases should be discussed in a multidisciplinary board.
Question 4: How can MDT discussion and whole-process management be better applied in the diagnosis and treatment of advanced GIST?
Emerson Y. Chen: As your team have done, it is always a conversation between thinking about the TKI controlling systemic disease but also treating definitively with surgery (and avoiding surgeries that could be too morbid for the patient).
Olugbenga Olowokure: Would encourage regular at least once a month multidisciplinary GI tumor board involving, surgical oncology team, pathology, radiology, and medical oncology where cases can be discussed.
Alessandro Mazzocca: MDT discussion should be considered in every patient with advanced GIST, particularly in those with oligometastatic disease and liver only metastasis. Different time point can be considered for discussion: diagnosis, disease response, disease oligo-progression.
Acknowledgments
The authors appreciate the academic support from the AME Gastrointestinal Stromal Tumor Collaborative Group.
Funding: The study was supported by the Scientific Research Fund of Yunnan Provincial Education Department (No. 2022J0227), and the Joint Special Funds for the Department of Science and Technology of Yunnan Province-Kunming Medical University (No. 202201AY070001-149).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-990/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-990/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ding H, Yu X, Yu Y, et al. Clinical significance of the molecular heterogeneity of gastrointestinal stromal tumors and related research: A systematic review. Oncol Rep 2020;43:751-64. [Crossref] [PubMed]
- Wang MX, Devine C, Segaran N, et al. Current update on molecular cytogenetics, diagnosis and management of gastrointestinal stromal tumors. World J Gastroenterol 2021;27:7125-33. [Crossref] [PubMed]
- Joyon N, Dumortier J, Aline-Fardin A, et al. Gastrointestinal stromal tumors (GIST) presenting in the liver: Diagnostic, prognostic and therapeutic issues. Clin Res Hepatol Gastroenterol 2018;42:e23-8. [Crossref] [PubMed]
- Dematteo RP, Gold JS, Saran L, et al. Tumor mitotic rate, size, and location independently predict recurrence after resection of primary gastrointestinal stromal tumor (GIST). Cancer 2008;112:608-15. [Crossref] [PubMed]
- Rare Tumors Gi Group. Comprehensive review into the challenges of gastrointestinal tumors in the Gulf and Levant countries. World J Clin Cases 2020;8:487-503. [Crossref] [PubMed]
- Chinese Gastrointestinal Stromal Tumor Expert Group. Consensus on the diagnosis and treatment of gastrointestinal stromal tumors in China (2008 edition). Chinese Clinical Oncology 2009;14:746-54.
- Chinese CSCO Gastrointestinal Stromal Tumor Expert Committee. Expert consensus on the diagnosis and treatment of gastrointestinal stromal tumors in China (2011 edition). Chinese Journal of Gastrointestinal Surgery 2012;15:301-7.
- Shen L, Li J, Qin S, et al. Consensus on the diagnosis and treatment of gastrointestinal stromal tumors in China (2013 edition). Chinese Clinical Oncology 2013;18:1025-32.
- Shen L, Cao H, Qin S, et al. Chinese consensus guidelines for diagnosis and management of gastrointestinal stromal tumor (2017 edition). Journal of Multidisciplinary Cancer Management 2018;4:31-43. (Electronic Version).
- Cao H, Tao K, Zhang P, et al. Chinese expert consensus on whole-process management of gastrointestinal stromal tumor (2020 edition). Chinese Journal of Practical Surgery 2020;40:1109-19.
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- General Assembly of the World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. J Am Coll Dent 2014;81:14-8. [PubMed]
- WHO Classification of Tumours Editorial Board. Digestive system tumours. Lyon: International Agency for Research on Cancer, 2019.
- Huss S, Pasternack H, Ihle MA, et al. Clinicopathological and molecular features of a large cohort of gastrointestinal stromal tumors (GISTs) and review of the literature: BRAF mutations in KIT/PDGFRA wild-type GISTs are rare events. Hum Pathol 2017;62:206-14. [Crossref] [PubMed]
- Yasui M, Tsujinaka T, Mori M, et al. Characteristics and prognosis of rectal gastrointestinal stromal tumors: an analysis of registry data. Surg Today 2017;47:1188-94. [Crossref] [PubMed]
- Guo W, Yang Z, Wei Y, et al. Radical excision versus local resection for primary rectal gastrointestinal stromal tumors. Cohort Study. Int J Surg 2020;77:190-7. [Crossref] [PubMed]
- Cavnar MJ, Seier K, Curtin C, et al. Outcome of 1000 Patients With Gastrointestinal Stromal Tumor (GIST) Treated by Surgery in the Pre- and Post-imatinib Eras. Ann Surg 2021;273:128-38. [Crossref] [PubMed]
- Corless CL, Ballman KV, Antonescu CR, et al. Pathologic and molecular features correlate with long-term outcome after adjuvant therapy of resected primary GI stromal tumor: the ACOSOG Z9001 trial. J Clin Oncol 2014;32:1563-70. Erratum in: J Clin Oncol 2014;32:3462. [Crossref] [PubMed]
- Raut CP, Espat NJ, Maki RG, et al. Efficacy and Tolerability of 5-Year Adjuvant Imatinib Treatment for Patients With Resected Intermediate- or High-Risk Primary Gastrointestinal Stromal Tumor: The PERSIST-5 Clinical Trial. JAMA Oncol 2018;4:e184060. [Crossref] [PubMed]
- Roberts PJ, Eisenberg B. Clinical presentation of gastrointestinal stromal tumors and treatment of operable disease. Eur J Cancer 2002;38:S37-8. [Crossref] [PubMed]
- Gomez D, Al-Mukthar A, Menon KV, et al. Aggressive surgical resection for the management of hepatic metastases from gastrointestinal stromal tumours: a single centre experience. HPB (Oxford) 2007;9:64-70. [Crossref] [PubMed]
- Zhu J, Yang Y, Zhou L, et al. A long-term follow-up of the imatinib mesylate treatment for the patients with recurrent gastrointestinal stromal tumor (GIST): the liver metastasis and the outcome. BMC Cancer 2010;10:199. [Crossref] [PubMed]
- Brudvik KW, Patel SH, Roland CL, et al. Survival After Resection of Gastrointestinal Stromal Tumor and Sarcoma Liver Metastases in 146 Patients. J Gastrointest Surg 2015;19:1476-83. [Crossref] [PubMed]
- Nunobe S, Sano T, Shimada K, et al. Surgery including liver resection for metastatic gastrointestinal stromal tumors or gastrointestinal leiomyosarcomas. Jpn J Clin Oncol 2005;35:338-41. [Crossref] [PubMed]
- Turley RS, Peng PD, Reddy SK, et al. Hepatic resection for metastatic gastrointestinal stromal tumors in the tyrosine kinase inhibitor era. Cancer 2012;118:3571-8. [Crossref] [PubMed]
- Xiao B, Peng J, Tang J, et al. Liver surgery prolongs the survival of patients with gastrointestinal stromal tumor liver metastasis: a retrospective study from a single center. Cancer Manag Res 2018;10:6121-7. [Crossref] [PubMed]
- Shi YN, Li Y, Wang LP, et al. Gastrointestinal stromal tumor (GIST) with liver metastases: An 18-year experience from the GIST cooperation group in North China. Medicine (Baltimore) 2017;96:e8240. [Crossref] [PubMed]
- Seesing MF, Tielen R, van Hillegersberg R, et al. Resection of liver metastases in patients with gastrointestinal stromal tumors in the imatinib era: A nationwide retrospective study. Eur J Surg Oncol 2016;42:1407-13. [Crossref] [PubMed]
- Renberg S, Zhang Y, Karlsson F, et al. The role of neoadjuvant imatinib in gastrointestinal stromal tumor patients: 20 years of experience from a tertial referral center. Int J Cancer 2022;151:906-13. [Crossref] [PubMed]
- Zhang X, Zhou Y, Wu X, et al. Cytoreductive surgery for metastatic gastrointestinal stromal tumors followed by sunitinib compared to followed by imatinib-a multi-center cohort study. Eur J Surg Oncol 2019;45:318-23. [Crossref] [PubMed]
- Houk BE, Bello CL, Poland B, et al. Relationship between exposure to sunitinib and efficacy and tolerability endpoints in patients with cancer: results of a pharmacokinetic/pharmacodynamic meta-analysis. Cancer Chemother Pharmacol 2010;66:357-71. [Crossref] [PubMed]
- Machairas N, Prodromidou A, Molmenti E, et al. Management of liver metastases from gastrointestinal stromal tumors: where do we stand? J Gastrointest Oncol 2017;8:1100-8. [Crossref] [PubMed]


